Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease
- PMID: 25389906
- PMCID: PMC4351594
- DOI: 10.1164/rccm.201406-1162OC
Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease
Abstract
Rationale: Asymptomatic relatives of patients with familial interstitial pneumonia (FIP), the inherited form of idiopathic interstitial pneumonia, carry increased risk for developing interstitial lung disease.
Objectives: Studying these at-risk individuals provides a unique opportunity to investigate early stages of FIP pathogenesis and develop predictive models of disease onset.
Methods: Seventy-five asymptomatic first-degree relatives of FIP patients (mean age, 50.8 yr) underwent blood sampling and high-resolution chest computed tomography (HRCT) scanning in an ongoing cohort study; 72 consented to bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsies. Twenty-seven healthy individuals were used as control subjects.
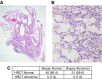
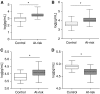
Measurements and main results: Eleven of 75 at-risk subjects (14%) had evidence of interstitial changes by HRCT, whereas 35.2% had abnormalities on transbronchial biopsies. No differences were noted in inflammatory cells in BAL between at-risk individuals and control subjects. At-risk subjects had increased herpesvirus DNA in cell-free BAL and evidence of herpesvirus antigen expression in alveolar epithelial cells (AECs), which correlated with expression of endoplasmic reticulum stress markers in AECs. Peripheral blood mononuclear cell and AEC telomere length were shorter in at-risk individuals than healthy control subjects. The minor allele frequency of the Muc5B rs35705950 promoter polymorphism was increased in at-risk subjects. Levels of several plasma biomarkers differed between at-risk subjects and control subjects, and correlated with abnormal HRCT scans.
Conclusions: Evidence of lung parenchymal remodeling and epithelial dysfunction was identified in asymptomatic individuals at risk for FIP. Together, these findings offer new insights into the early pathogenesis of idiopathic interstitial pneumonia and provide an ongoing opportunity to characterize presymptomatic abnormalities that predict progression to clinical disease.
Keywords: IPF; alveolar epithelial cell; biomarker; bronchoscopy; telomere.
Figures




Comment in
-
A first glimpse at the early origins of idiopathic pulmonary fibrosis.Am J Respir Crit Care Med. 2015 Feb 15;191(4):366-8. doi: 10.1164/rccm.201412-2187ED. Am J Respir Crit Care Med. 2015. PMID: 25679101 Free PMC article. No abstract available.
References
-
- García-Sancho C, Buendía-Roldán I, Fernández-Plata MR, Navarro C, Pérez-Padilla R, Vargas MH, Loyd JE, Selman M. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105:1902–1907. - PubMed
-
- Loyd JE. Pulmonary fibrosis in families. Am J Respir Cell Mol Biol. 2003;29(3) Suppl:S47–S50. - PubMed
MeSH terms
Substances
Grants and funding
- P01 HL092870/HL/NHLBI NIH HHS/United States
- R01 HL109118/HL/NHLBI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- U01 HG007674/HG/NHGRI NIH HHS/United States
- R01 HL105479/HL/NHLBI NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- R01 HL085317/HL/NHLBI NIH HHS/United States
- I01 BX001988/BX/BLRD VA/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- K08 HL085406/HL/NHLBI NIH HHS/United States
- R01 HL088263/HL/NHLBI NIH HHS/United States
- R01 HL119503/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

