A polymeric prodrug of 5-fluorouracil-1-acetic acid using a multi-hydroxyl polyethylene glycol derivative as the drug carrier
- PMID: 25389968
- PMCID: PMC4229301
- DOI: 10.1371/journal.pone.0112888
A polymeric prodrug of 5-fluorouracil-1-acetic acid using a multi-hydroxyl polyethylene glycol derivative as the drug carrier
Abstract
Purpose: Macromolecular prodrugs obtained by covalently conjugating small molecular drugs with polymeric carriers were proven to accomplish controlled and sustained release of the therapeutic agents in vitro and in vivo. Polyethylene glycol (PEG) has been extensively used due to its low toxicity, low immunogenicity and high biocompatibility. However, for linear PEG macromolecules, the number of available hydroxyl groups for drug coupling does not change with the length of polymeric chain, which limits the application of PEG for drug conjugation purposes. To increase the drug loading and prolong the retention time of 5-fluorouracil (5-Fu), a macromolecular prodrug of 5-Fu, 5-fluorouracil-1 acid-PAE derivative (5-FA-PAE) was synthesized and tested for the antitumor activity in vivo.
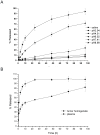
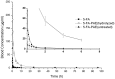
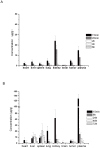
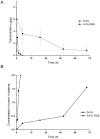
Methods: PEG with a molecular weight of 38 kDa was selected to synthesize the multi-hydroxyl polyethylene glycol derivative (PAE) through an addition reaction. 5-fluorouracil-1 acetic acid (5-FA), a 5-Fu derivative was coupled with PEG derivatives via ester bond to form a macromolecular prodrug, 5-FA-PAE. The in vitro drug release, pharmacokinetics, in vivo distribution and antitumor effect of the prodrug were investigated, respectively.
Results: The PEG-based prodrug obtained in this study possessed an exceedingly high 5-FA loading efficiency of 10.58%, much higher than the maximum drug loading efficiency of unmodified PEG with the same molecular weight, which was 0.98% theoretically. Furthermore, 5-FA-PAE exhibited suitable sustained release in tumors.
Conclusion: This study provides a new approach for the development of the delivery to tumors of anticancer agents with PEG derivatives.
Conflict of interest statement
Figures

Similar articles
-
Preparation and characterization of 5-fluorouracil-loaded PLLA-PEG/PEG nanoparticles by a novel supercritical CO2 technique.Int J Pharm. 2012 Oct 15;436(1-2):272-81. doi: 10.1016/j.ijpharm.2012.06.022. Epub 2012 Jun 18. Int J Pharm. 2012. PMID: 22721846
-
Cytocompatible chitosan-graft-mPEG-based 5-fluorouracil-loaded polymeric nanoparticles for tumor-targeted drug delivery.Drug Dev Ind Pharm. 2018 Mar;44(3):365-376. doi: 10.1080/03639045.2017.1371741. Epub 2017 Dec 5. Drug Dev Ind Pharm. 2018. PMID: 28835136
-
Tumor-targeting peptide conjugated pH-responsive micelles as a potential drug carrier for cancer therapy.Bioconjug Chem. 2010 Feb 17;21(2):208-13. doi: 10.1021/bc9005283. Bioconjug Chem. 2010. PMID: 20073455
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs.Adv Drug Deliv Rev. 2004 May 7;56(9):1273-89. doi: 10.1016/j.addr.2003.12.004. Adv Drug Deliv Rev. 2004. PMID: 15109769 Review.
Cited by
-
Smart and Biomimetic 3D and 4D Printed Composite Hydrogels: Opportunities for Different Biomedical Applications.Biomedicines. 2021 Oct 26;9(11):1537. doi: 10.3390/biomedicines9111537. Biomedicines. 2021. PMID: 34829766 Free PMC article. Review.
-
Synthesis, Characterization and DNA Binding Investigations of a New Binuclear Ag(I) Complex and Evaluation of Its Anticancer Property.Int J Nanomedicine. 2020 Feb 12;15:953-964. doi: 10.2147/IJN.S225038. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32103949 Free PMC article.
-
Covalently mucoadhesive amphiphilic prodrug of 5-fluorouracil for enhanced permeation and improved oral absorption.Drug Deliv Transl Res. 2018 Jun;8(3):645-656. doi: 10.1007/s13346-018-0502-z. Drug Deliv Transl Res. 2018. PMID: 29532356
-
In vitro and in vivo evaluation of macromolecular prodrug GC-FUA based nanoparticle for hepatocellular carcinoma chemotherapy.Drug Deliv. 2017 Nov;24(1):459-466. doi: 10.1080/10717544.2016.1264499. Drug Deliv. 2017. PMID: 28219253 Free PMC article.
-
Synthesis and DNA/RNA complementation studies of peptide nucleic acids containing 5-halouracils.Medchemcomm. 2016 Dec 12;8(2):385-389. doi: 10.1039/c6md00536e. eCollection 2017 Feb 1. Medchemcomm. 2016. PMID: 30108754 Free PMC article.
References
-
- Sarkar FH (2010) Recent trends in anti-cancer drug discovery. Mini Rev Med Chem 10: 357–358. - PubMed
-
- Na Y (2009) Recent cancer drug development with xanthone structures. J Pharm Pharmacol 61: 707–712. - PubMed
-
- Meada H (2001) SMANCS and polymer-conjugates macromolecular drug: advantages in cancer chemotherapy. Adv Drug Deliv Rev 46: 169–185. - PubMed
-
- Dang CT (2006) Drug treatments for adjuvant chemotherapy in breast cancer: recent trials and future directions. Expert Rev Anticancer Ther 6: 427–436. - PubMed
-
- Thompson N, Lyons J (2005) Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol 5: 350–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

