Tissue-specific expression of monocarboxylate transporters during fasting in mice
- PMID: 25390336
- PMCID: PMC4229183
- DOI: 10.1371/journal.pone.0112118
Tissue-specific expression of monocarboxylate transporters during fasting in mice
Abstract
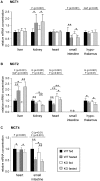
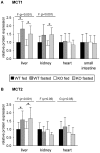
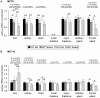
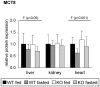
Monocarboxylates such as pyruvate, lactate and ketone bodies are crucial for energy supply of all tissues, especially during energy restriction. The transport of monocarboxylates across the plasma membrane of cells is mediated by monocarboxylate transporters (MCTs). Out of 14 known mammalian MCTs, six isoforms have been functionally characterized to transport monocarboxylates and short chain fatty acids (MCT1-4), thyroid hormones (MCT8, -10) and aromatic amino acids (MCT10). Knowledge on the regulation of the different MCT isoforms is rare. In an attempt to get more insights in regulation of MCT expression upon energy deprivation, we carried out a comprehensive analysis of tissue specific expression of five MCT isoforms upon 48 h of fasting in mice. Due to the crucial role of peroxisome proliferator-activated receptor (PPAR)-α as a central regulator of energy metabolism and as known regulator of MCT1 expression, we included both wildtype (WT) and PPARα knockout (KO) mice in our study. Liver, kidney, heart, small intestine, hypothalamus, pituitary gland and thyroid gland of the mice were analyzed. Here we show that the expression of all examined MCT isoforms was markedly altered by fasting compared to feeding. Expression of MCT1, MCT2 and MCT10 was either increased or decreased by fasting dependent on the analyzed tissue. MCT4 and MCT8 were down-regulated by fasting in all examined tissues. However, PPARα appeared to have a minor impact on MCT isoform regulation. Due to the fundamental role of MCTs in transport of energy providing metabolites and hormones involved in the regulation of energy homeostasis, we assumed that the observed fasting-induced adaptations of MCT expression seem to ensure an adequate energy supply of tissues during the fasting state. Since, MCT isoforms 1-4 are also necessary for the cellular uptake of drugs, the fasting-induced modifications of MCT expression have to be considered in future clinical care algorithms.
Conflict of interest statement
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

