Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits
- PMID: 25390368
- PMCID: PMC4229098
- DOI: 10.1371/journal.pone.0111898
Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits
Abstract
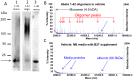
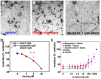
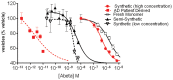
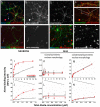
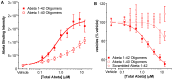
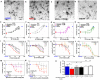
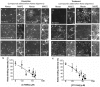
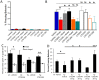
Synaptic dysfunction and loss caused by age-dependent accumulation of synaptotoxic beta amyloid (Abeta) 1-42 oligomers is proposed to underlie cognitive decline in Alzheimer's disease (AD). Alterations in membrane trafficking induced by Abeta oligomers mediates reduction in neuronal surface receptor expression that is the basis for inhibition of electrophysiological measures of synaptic plasticity and thus learning and memory. We have utilized phenotypic screens in mature, in vitro cultures of rat brain cells to identify small molecules which block or prevent the binding and effects of Abeta oligomers. Synthetic Abeta oligomers bind saturably to a single site on neuronal synapses and induce deficits in membrane trafficking in neuronal cultures with an EC50 that corresponds to its binding affinity. The therapeutic lead compounds we have found are pharmacological antagonists of Abeta oligomers, reducing the binding of Abeta oligomers to neurons in vitro, preventing spine loss in neurons and preventing and treating oligomer-induced deficits in membrane trafficking. These molecules are highly brain penetrant and prevent and restore cognitive deficits in mouse models of Alzheimer's disease. Counter-screening these compounds against a broad panel of potential CNS targets revealed they are highly potent and specific ligands of the sigma-2/PGRMC1 receptor. Brain concentrations of the compounds corresponding to greater than 80% receptor occupancy at the sigma-2/PGRMC1 receptor restore cognitive function in transgenic hAPP Swe/Ldn mice. These studies demonstrate that synthetic and human-derived Abeta oligomers act as pharmacologically-behaved ligands at neuronal receptors--i.e. they exhibit saturable binding to a target, they exert a functional effect related to their binding and their displacement by small molecule antagonists blocks their functional effect. The first-in-class small molecule receptor antagonists described here restore memory to normal in multiple AD models and sustain improvement long-term, representing a novel mechanism of action for disease-modifying Alzheimer's therapeutics.
Conflict of interest statement
Figures





References
-
- Haes AJ, Chang L, Klein WL, Van Duyne RP (2005) Detection of a biomarker for Alzheimer's disease from synthetic and clinical samples using a nanoscale optical biosensor. J Am Chem Soc 127: 2264–2271. - PubMed
-
- Bao F, Wicklund L, Lacor PN, Klein WL, Nordberg A, et al.. (2012) Different beta-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol Aging 33: : 825 e821–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

