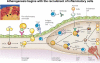
The core mechanism of dry eye disease is inflammation
- PMID: 25390549
- PMCID: PMC4231828
- DOI: 10.1097/ICL.0000000000000042
The core mechanism of dry eye disease is inflammation
Erratum in
- Eye Contact Lens. 2014 Sep;40(5):311
Abstract
Purpose: The purpose of this article is to review the evidence for the hypothesis that the core mechanism of dry eye disease (DED) is inflammation, including evidence from recent basic, clinical, and translational research involving human patients, animal models, and cell cultures.
Methods: Using the key words "dry eye + inflammation," the authors conducted a comprehensive search of the PubMed and Web of Science databases for scientific articles published in English between January 1, 1900 and August 30, 2013 on the role of inflammation in DED in cell cultures, animal models, and humans. The resulting articles were then categorized and reviewed.
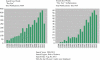
Results: The literature search revealed a total of 458 publications, almost all published after 1992. The percentages of original studies and review articles are 77.29% (354) and 22.71% (104), respectively. Among the original studies, the number of reports on human DED is 200 (43.7%), on animal models is 115 (25.1%), and cell cultures is 39 (8.5%). A yearly distributing plot revealed that 76% were published from 2003 to 2011, 53% from 2008 to 2012, and 11% during the first 9 months of 2013. This distribution signifies a rapidly growing awareness of the importance of inflammation in DED pathogenesis.
Conclusions: Inflammation plays a key role in the pathogenesis of DED as evidenced by research using tissue culture, animal models, and subjects with DED. Developing biomarkers for inflammation of the ocular surface will provide improved understanding of the mechanisms leading to DED, classification of the severity of DED, and objective metrics for outcome measures of treatment. The chronicity of the disease suggests that dysregulation of immune mechanisms leads to a cycle of continued inflammation, accompanied by alterations in both innate and adaptive immune responses. Given the underlying mechanism for DED, developing effective and safe anti-inflammatory treatments is likely to be beneficial for patients with DED.
Figures

References
-
- DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92. - PubMed
-
- Pflugfelder SC, Stern ME. Immunoregulation on the ocular surface: 2nd Cullen Symposium. Ocul Surf. 2009;7:67–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

