Prodrugs of phosphonates and phosphates: crossing the membrane barrier
- PMID: 25391982
- PMCID: PMC4774048
- DOI: 10.1007/128_2014_561
Prodrugs of phosphonates and phosphates: crossing the membrane barrier
Abstract
A substantial portion of metabolism involves transformation of phosphate esters, including pathways leading to nucleotides and oligonucleotides, carbohydrates, isoprenoids and steroids, and phosphorylated proteins. Because the natural substrates bear one or more negative charges, drugs that target these enzymes generally must be charged as well, but small charged molecules can have difficulty traversing the cell membrane by means other than endocytosis. The resulting dichotomy has stimulated a great deal of effort to develop effective prodrugs, compounds that carry little or no charge to enable them to transit biological membranes, but able to release the parent drug once inside the target cell. This chapter presents recent studies on advances in prodrug forms, along with representative examples of their application to marketed and developmental drugs.
Figures
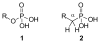
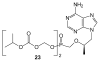
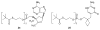
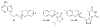
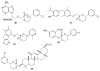
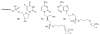
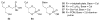

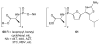
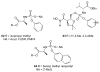
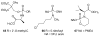


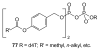


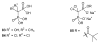
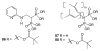
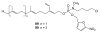
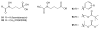

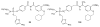
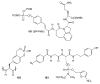
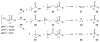
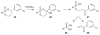
References
-
- Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–8. - PubMed
-
- Engel R. Phosphonates as analogs of natural phosphates. Chem Rev. 1977;77:349–67.
-
- Williams R, Jencks WP, Westheimer FH. Table of pKa values. http://research.chem.psu.edu/brpgroup/pKa_compilation.pdf.
-
- Wiemer DF. Synthesis of nonracemic phosphonates. Tetrahedron. 1997;53:16609–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

