Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis
- PMID: 25392011
- PMCID: PMC4278923
- DOI: 10.1128/CVI.00466-14
Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis
Abstract
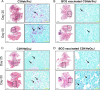
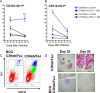
The global epidemic caused by the bacterial pathogen Mycobacterium tuberculosis continues unabated. Moreover, the only available vaccine against tuberculosis, Mycobacterium bovis bacillus Calmette-Guérin (BCG), demonstrates variable efficacy. To respond to this global threat, new animal models that mimic the pathological disease process in humans are required for vaccine testing. One new model, susceptible C3Heb/FeJ mice, is similar to human tuberculosis in that these animals are capable of forming necrotic tubercle granulomas, in contrast to resistant C3H/HeOuJ mice. In this study, we evaluated the impact of prior BCG vaccination of C3Heb/FeJ and C3H/HeOuJ mice on exposure to a low-dose aerosol of Mycobacterium tuberculosis W-Beijing strain SA161. Both BCG-vaccinated murine strains demonstrated reduced bacterial loads 25 days after infection compared to controls, indicating vaccine efficacy. However, during chronic infection, vaccine efficacy waned in C3H/HeOuJ but not in C3Heb/FeJ mice. Protection in vaccinated C3Heb/FeJ mice was associated with reduced numbers of CD11b(+) Gr1(+) cells, increased numbers of effector and memory T cells, and an absence of necrotic granulomas. BCG vaccine efficacy waned in C3H/HeOuJ mice, as indicated by reduced expression of gamma interferon (IFN-γ) and increased expressions of interleukin-17 (IL-17), IL-10, and Foxp3 by T cells compared to C3Heb/FeJ mice. This is the first murine vaccine model system described to date that can be utilized to dissect differential vaccine-derived immune efficacy.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- World Health Organization. 2011. Global tuberculosis control: WHO report 2011, p viii, 122–246. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

