Measles virus glycoprotein complexes preassemble intracellularly and relax during transport to the cell surface in preparation for fusion
- PMID: 25392208
- PMCID: PMC4300639
- DOI: 10.1128/JVI.02754-14
Measles virus glycoprotein complexes preassemble intracellularly and relax during transport to the cell surface in preparation for fusion
Abstract
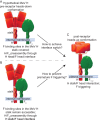
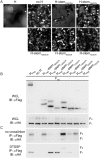
Measles virus (MeV), a morbillivirus within the paramyxovirus family, expresses two envelope glycoproteins. The attachment (H) protein mediates receptor binding, followed by triggering of the fusion (F) protein, which leads to merger of the viral envelope with target cell membranes. Receptor binding by members of related paramyxovirus genera rearranges the head domains of the attachment proteins, liberating an F-contact domain within the attachment protein helical stalk. However, morbillivirus glycoproteins first assemble intracellularly prior to receptor binding, raising the question of whether alternative protein-protein interfaces are involved or whether an entirely distinct triggering principle is employed. To test these possibilities, we generated headless H stem mutants of progressively shorter length. Conformationally restricted H stems remained capable of intracellular assembly with a standard F protein and a soluble MeV F mutant. Proteolytic maturation of F, but not the altered biochemical conditions at the cell surface, reduces the strength of glycoprotein interaction, readying the complexes for triggering. F mutants stabilized in the prefusion conformation interact with H intracellularly and at the cell surface, while destabilized F mutants interact only intracellularly, prior to F maturation. These results showcase an MeV entry machinery that functionally varies conserved motifs of the proposed paramyxovirus infection pathway. Intracellular and plasma membrane-resident MeV glycoprotein complexes employ the same protein-protein interface. F maturation prepares for complex separation after triggering, and the H head domains in prereceptor-bound conformation prevent premature stalk rearrangements and F activation. Intracellular preassembly affects MeV fusion profiles and may contribute to the high cell-to-cell fusion activity characteristic of the morbillivirus genus.
Importance: Paramyxoviruses of the morbillivirus genus, such as measles, are highly contagious, major human and animal pathogens. MeV envelope glycoproteins preassemble intracellularly into tightly associated hetero-oligomers. To address whether preassembly reflects a unique measles virus entry strategy, we characterized the protein-protein interface of intracellular and surface-exposed fusion complexes and investigated the effect of the attachment protein head domains, glycoprotein maturation, and altered biochemical conditions at the cell surface on measles virus fusion complexes. Our results demonstrate that measles virus functionally varies conserved elements of the paramyxovirus entry pathway, providing a possible explanation for the high cell-to-cell fusion activity of morbilliviruses. Insight gained from these data affects the design of effective broad-spectrum paramyxovirus entry inhibitors.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
A stabilized headless measles virus attachment protein stalk efficiently triggers membrane fusion.J Virol. 2013 Nov;87(21):11693-703. doi: 10.1128/JVI.01945-13. Epub 2013 Aug 21. J Virol. 2013. PMID: 23966411 Free PMC article.
-
A Structurally Unresolved Head Segment of Defined Length Favors Proper Measles Virus Hemagglutinin Tetramerization and Efficient Membrane Fusion Triggering.J Virol. 2015 Oct 7;90(1):68-75. doi: 10.1128/JVI.02253-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26446605 Free PMC article.
-
Regulatory Role of the Morbillivirus Attachment Protein Head-to-Stalk Linker Module in Membrane Fusion Triggering.J Virol. 2018 Aug 29;92(18):e00679-18. doi: 10.1128/JVI.00679-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29997204 Free PMC article.
-
Structural and mechanistic studies of measles virus illuminate paramyxovirus entry.PLoS Pathog. 2011 Jun;7(6):e1002058. doi: 10.1371/journal.ppat.1002058. Epub 2011 Jun 2. PLoS Pathog. 2011. PMID: 21655106 Free PMC article. Review.
-
Measles virus glycoprotein complex assembly, receptor attachment, and cell entry.Curr Top Microbiol Immunol. 2009;329:59-76. doi: 10.1007/978-3-540-70523-9_4. Curr Top Microbiol Immunol. 2009. PMID: 19198562 Free PMC article. Review.
Cited by
-
Probing Morbillivirus Antisera Neutralization Using Functional Chimerism between Measles Virus and Canine Distemper Virus Envelope Glycoproteins.Viruses. 2019 Jul 27;11(8):688. doi: 10.3390/v11080688. Viruses. 2019. PMID: 31357579 Free PMC article.
-
The DI-DII linker of human parainfluenza virus type 3 fusion protein is critical for the virus.Virus Genes. 2020 Feb;56(1):37-48. doi: 10.1007/s11262-019-01713-8. Epub 2019 Nov 25. Virus Genes. 2020. PMID: 31768710
-
Receptor-mediated cell entry of paramyxoviruses: Mechanisms, and consequences for tropism and pathogenesis.J Biol Chem. 2020 Feb 28;295(9):2771-2786. doi: 10.1074/jbc.REV119.009961. Epub 2020 Jan 16. J Biol Chem. 2020. PMID: 31949044 Free PMC article. Review.
-
Suppression of viral RNA polymerase activity is necessary for persistent infection during the transformation of measles virus into SSPE virus.PLoS Pathog. 2023 Jul 26;19(7):e1011528. doi: 10.1371/journal.ppat.1011528. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37494386 Free PMC article.
-
Multiple Strategies Reveal a Bidentate Interaction between the Nipah Virus Attachment and Fusion Glycoproteins.J Virol. 2016 Nov 14;90(23):10762-10773. doi: 10.1128/JVI.01469-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654290 Free PMC article.
References
-
- Griffin DE. 2007. Measles virus, p 1551–1585 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Lamb RA, Parks GD. 2007. Paramyxoviridae: the viruses and their replication, p 1449–1496 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

