Cellular origins of cold-induced brown adipocytes in adult mice
- PMID: 25392270
- PMCID: PMC4285542
- DOI: 10.1096/fj.14-263038
Cellular origins of cold-induced brown adipocytes in adult mice
Abstract
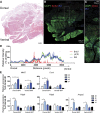
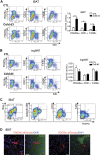
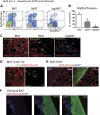
This work investigated how cold stress induces the appearance of brown adipocytes (BAs) in brown and white adipose tissues (WATs) of adult mice. In interscapular brown adipose tissue (iBAT), cold exposure increased proliferation of endothelial cells and interstitial cells expressing platelet-derived growth factor receptor, α polypeptide (PDGFRα) by 3- to 4-fold. Surprisingly, brown adipogenesis and angiogenesis were largely restricted to the dorsal edge of iBAT. Although cold stress did not increase proliferation in inguinal white adipose tissue (ingWAT), the percentage of BAs, defined as multilocular adipocytes that express uncoupling protein 1, rose from undetectable to 30% of total adipocytes. To trace the origins of cold-induced BAs, we genetically tagged PDGFRα(+) cells and adipocytes prior to cold exposure, using Pdgfra-Cre recombinase estrogen receptor T2 fusion protein (CreER(T2)) and adiponectin-CreER(T2), respectively. In iBAT, cold stress triggered the proliferation and differentiation of PDGFRα(+) cells into BAs. In contrast, all newly observed BAs in ingWAT (5207 out of 5207) were derived from unilocular adipocytes tagged by adiponectin-CreER(T2)-mediated recombination. Surgical denervation of iBAT reduced cold-induced brown adipogenesis by >85%, whereas infusion of norepinephrine (NE) mimicked the effects of cold in warm-adapted mice. NE-induced de novo brown adipogenesis in iBAT was eliminated in mice lacking β1-adrenergic receptors. These observations identify a novel tissue niche for brown adipogenesis in iBAT and further define depot-specific mechanisms of BA recruitment.
Keywords: Myf5; adipogenesis; innervation; lineage tracing; tyrosine hydroxylase.
© FASEB.
Figures





References
-
- Cannon B., Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 - PubMed
-
- Gesta S., Tseng Y. H., Kahn C. R. (2007) Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 - PubMed
-
- Himms-Hagen J. (1990) Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 4, 2890–2898 - PubMed
-
- Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., Eychmüller A., Gordts P. L., Rinninger F., Bruegelmann K., Freund B., Nielsen P., Merkel M., Heeren J. (2011) Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

