Reversible behavioral phenotypes in a conditional mouse model of TDP-43 proteinopathies
- PMID: 25392493
- PMCID: PMC4298649
- DOI: 10.1523/JNEUROSCI.1918-14.2014
Reversible behavioral phenotypes in a conditional mouse model of TDP-43 proteinopathies
Abstract
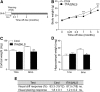
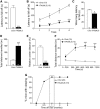
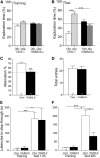
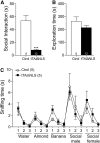
Transactive response DNA-binding protein 43 (TDP-43) mislocalization and aggregation are hallmark features of amyotrophic lateral sclerosis and frontotemporal dementia (FTD). We have previously shown in mice that inducible overexpression of a cytoplasmically localized form of TDP-43 (TDP-43-ΔNLS) in forebrain neurons evokes neuropathological changes that recapitulate several features of TDP-43 proteinopathies. Detailed behavioral phenotyping could provide further validation for its usage as a model for FTD. In the present study, we performed a battery of behavioral tests to evaluate motor, cognitive, and social phenotypes in this model. We found that transgene (Tg) induction by doxycycline removal at weaning led to motor abnormalities including hyperlocomotion in the open field test, impaired coordination and balance in the rotarod test, and increased spasticity as shown by a clasping phenotype. Cognitive assessment demonstrated impaired recognition and spatial memory, measured by novel object recognition and Y-maze tests. Remarkably, TDP-43-ΔNLS mice displayed deficits in social behavior, mimicking a key aspect of FTD. To determine whether these symptoms were reversible, we suppressed Tg expression for 14 d in 1.5-month-old mice showing an established behavioral phenotype but modest neurodegeneration and found that motor and cognitive deficits were ameliorated; however, social performance remained altered. When Tg expression was suppressed in 6.5-month-old mice showing overt neurodegeneration, motor deficits were irreversible. These results indicate that TDP-43-ΔNLS mice display several core behavioral features of FTD with motor neuron disease, possibly due to functional changes in surviving neurons, and might serve as a valuable tool to unveil the underlying mechanisms of this and other TDP-43 proteinopathies.
Keywords: Behavioral phenotypes; TDP-43; frontotemporal dementia; neurodegeneration; proteinopathies; transgenic mice.
Copyright © 2014 the authors 0270-6474/14/3415244-16$15.00/0.
Figures





References
-
- Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SS, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, Clare AJ, Badders NM, Bilican B, Chaum E, Chandran S, Shaw CE, Eggan KC, Maniatis T, Taylor JP. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
