Acetylation of Gly1 and Lys2 promotes aggregation of human γD-crystallin
- PMID: 25393041
- PMCID: PMC4245984
- DOI: 10.1021/bi501004y
Acetylation of Gly1 and Lys2 promotes aggregation of human γD-crystallin
Abstract
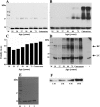
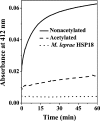
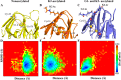
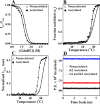
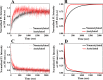
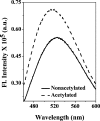
The human lens contains three major protein families: α-, β-, and γ-crystallin. Among the several variants of γ-crystallin in the human lens, γD-crystallin is a major form. γD-Crystallin is primarily present in the nuclear region of the lens and contains a single lysine residue at the second position (K2). In this study, we investigated the acetylation of K2 in γD-crystallin in aging and cataractous human lenses. Our results indicated that K2 is acetylated at an early age and that the amount of K2-acetylated γD-crystallin increased with age. Mass spectrometric analysis revealed that in addition to K2, glycine 1 (G1) was acetylated in γD-crystallin from human lenses and in γD-crystallin acetylated in vitro. The chaperone ability of α-crystallin for acetylated γD-crystallin was lower than that for the nonacetylated protein. The tertiary structure and the microenvironment of the cysteine residues were significantly altered by acetylation. The acetylated protein exhibited higher surface hydrophobicity, was unstable against thermal and chemical denaturation, and exhibited a higher propensity to aggregate at 80 °C in comparison to the nonacetylated protein. Acetylation enhanced the GdnHCl-induced unfolding and slowed the subsequent refolding of γD-crystallin. Theoretical analysis indicated that the acetylation of K2 and G1 reduced the structural stability of the protein and brought the distal cysteine residues (C18 and C78) into close proximity. Collectively, these results indicate that the acetylation of G1 and K2 residues in γD-crystallin likely induced a molten globule-like structure, predisposing it to aggregation, which may account for the high content of aggregated proteins in the nucleus of aged and cataractous human lenses.
Figures





References
-
- Bloemendal H. (1982) Lens proteins. CRC Crit. Rev. Biochem. 12, 1–38. - PubMed
-
- Benedek G. B. (1971) Theory of transparency of the eye. Appl. Opt. 10, 459–473. - PubMed
-
- Delaye M.; Tardieu A. (1983) Short-range order of Crystallin proteins accounts for eye lens transparency. Nature 302, 415–417. - PubMed
-
- Bron A. J.; Vrensen G. F.; Koretz J.; Maraini G.; Harding J. J. (2000) The ageing lens. Ophthalmologica 214, 86–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

