Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy
- PMID: 25393648
- PMCID: PMC4231123
- DOI: 10.1371/journal.ppat.1004473
Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy
Abstract
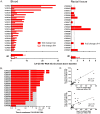
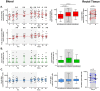
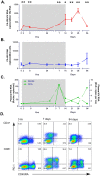
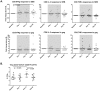
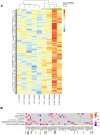
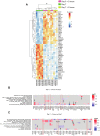
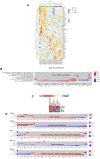
Human immunodeficiency virus (HIV) persistence in latently infected resting memory CD4+ T-cells is the major barrier to HIV cure. Cellular histone deacetylases (HDACs) are important in maintaining HIV latency and histone deacetylase inhibitors (HDACi) may reverse latency by activating HIV transcription from latently infected CD4+ T-cells. We performed a single arm, open label, proof-of-concept study in which vorinostat, a pan-HDACi, was administered 400 mg orally once daily for 14 days to 20 HIV-infected individuals on suppressive antiretroviral therapy (ART). The primary endpoint was change in cell associated unspliced (CA-US) HIV RNA in total CD4+ T-cells from blood at day 14. The study is registered at ClinicalTrials.gov (NCT01365065). Vorinostat was safe and well tolerated and there were no dose modifications or study drug discontinuations. CA-US HIV RNA in blood increased significantly in 18/20 patients (90%) with a median fold change from baseline to peak value of 7.4 (IQR 3.4, 9.1). CA-US RNA was significantly elevated 8 hours post drug and remained elevated 70 days after last dose. Significant early changes in expression of genes associated with chromatin remodeling and activation of HIV transcription correlated with the magnitude of increased CA-US HIV RNA. There were no statistically significant changes in plasma HIV RNA, concentration of HIV DNA, integrated DNA, inducible virus in CD4+ T-cells or markers of T-cell activation. Vorinostat induced a significant and sustained increase in HIV transcription from latency in the majority of HIV-infected patients. However, additional interventions will be needed to efficiently induce virus production and ultimately eliminate latently infected cells.
Trial registration: ClinicalTrials.gov NCT01365065.
Conflict of interest statement
JHE, JM and JR's institution has received funding for clinical research from Merck, Sharp & Dohme, Janssen-Cilag, Gilead Sciences, Bristol-Myers Squibb and ViiV Healthcare. JFH's institution has received funding for investigator-initiated research and service on Advisory Boards from Merck, Sharp & Dohme, Janssen-Cilag, Gilead Sciences, and ViiV Healthcare. JW has been a community member of a Merck Sharpe Dohme Advisory Board. MHP has undertaken paid consultancies for Merck. SGD has received grant support from Merck and Gilead. NC has received grant support from Merck. DJH is an employee of and has stock ownership in Merck Sharpe and Dohme. SRL's institution has received honoraria from Merck, Gilead, Viiv Healthcare, Janssen and Bristol Myers Squibb for participation in educational and consulting activities. She has received grants for investigator initiated research from Merck and Gilead. All other authors report no conflicts of interest.This does not alter our adherence to all PLOS policies on sharing data and materials.
Figures


References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, et al. (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278: 1295–1300. - PubMed
-
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, et al. (1997) Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387: 183–188. - PubMed
-
- Smith MZ, Wightman F, Lewin SR (2012) HIV reservoirs and strategies for eradication. Curr HIV/AIDS Rep 9: 5–15. - PubMed
-
- Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM (2004) Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS 18: 1101–1108. - PubMed
-
- Shehu-Xhilaga M, Rhodes D, Wightman F, Liu HB, Solomon A, et al. (2009) The novel histone deacetylase inhibitors metacept-1 and metacept-3 potently increase HIV-1 transcription in latently infected cells. AIDS 23: 2047–2050. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

