Biocompatible infinite-coordination-polymer nanoparticle-nucleic-acid conjugates for antisense gene regulation
- PMID: 25393766
- PMCID: PMC4314394
- DOI: 10.1002/anie.201407946
Biocompatible infinite-coordination-polymer nanoparticle-nucleic-acid conjugates for antisense gene regulation
Abstract
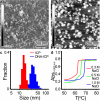
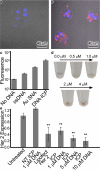
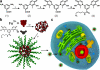
Herein, we report the synthesis of DNA-functionalized infinite-coordination-polymer (ICP) nanoparticles as biocompatible gene-regulation agents. ICP nanoparticles were synthesized from ferric nitrate and a ditopic 3-hydroxy-4-pyridinone (HOPO) ligand bearing a pendant azide. Addition of Fe(III) to a solution of the ligand produced nanoparticles, which were colloidally unstable in the presence of salts. Conjugation of DNA to the Fe(III)-HOPO ICP particles by copper-free click chemistry afforded colloidally stable nucleic-acid nanoconstructs. The DNA-ICP particles, when cross-linked through sequence-specific hybridization, exhibited narrow, highly cooperative melting transitions consistent with dense DNA surface loading. The ability of the DNA-ICP particles to enter cells and alter protein expression was also evaluated. Our results indicate that these novel particles carry nucleic acids into mammalian cells without the need for transfection agents and are capable of efficient gene knockdown.
Keywords: antisense gene regulation; coordination polymers; gene knockdown; iron; nanoparticles.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Zheng D, Seferos DS, Giljohann DA, Patel PC, Mirkin CA. Nano Lett. 2009;9:3258–3261. - PMC - PubMed
- Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. J. Am. Chem. Soc. 2007;129:5477. - PMC - PubMed
- Prigodich AE, Randeria PS, Briley WE, Kim NJ, Daniel WL, Giljohann DA, Mirkin CA. Anal. Chem. 2012;84:2062–2066. - PMC - PubMed
-
- Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

