Inactivation of human salivary glutathione transferase P1-1 by hypothiocyanite: a post-translational control system in search of a role
- PMID: 25393952
- PMCID: PMC4231102
- DOI: 10.1371/journal.pone.0112797
Inactivation of human salivary glutathione transferase P1-1 by hypothiocyanite: a post-translational control system in search of a role
Abstract
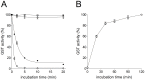
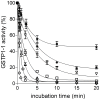
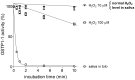
Glutathione transferases (GSTs) are a superfamily of detoxifying enzymes over-expressed in tumor tissues and tentatively proposed as biomarkers for localizing and monitoring injury of specific tissues. Only scarce and contradictory reports exist about the presence and the level of these enzymes in human saliva. This study shows that GSTP1-1 is the most abundant salivary GST isoenzyme, mainly coming from salivary glands. Surprisingly, its activity is completely obscured by the presence of a strong oxidizing agent in saliva that causes a fast and complete, but reversible, inactivation. Although salivary α-defensins are also able to inhibit the enzyme causing a peculiar half-site inactivation, a number of approaches (mass spectrometry, site directed mutagenesis, chromatographic and spectrophotometric data) indicated that hypothiocyanite is the main salivary inhibitor of GSTP1-1. Cys47 and Cys101, the most reactive sulfhydryls of GSTP1-1, are mainly involved in a redox interaction which leads to the formation of an intra-chain disulfide bridge. A reactivation procedure has been optimized and used to quantify GSTP1-1 in saliva of 30 healthy subjects with results of 42±4 mU/mg-protein. The present study represents a first indication that salivary GSTP1-1 may have a different and hitherto unknown function. In addition it fulfills the basis for future investigations finalized to check the salivary GSTP1-1 as a diagnostic biomarker for diseases.
Conflict of interest statement
Figures









References
-
- Amado FM, Ferreira RP, Vitorino R (2013) One decade of salivary proteomics: current approaches and outstanding challenges. Clin Biochem 46: 506–517. - PubMed
-
- Cabras T, Iavarone F, Manconi B, Olianas A, Sanna MT, et al. (2014) Top-down analytical platforms for the characterization of the human salivary proteome. Bioanalysis 6: 563–581. - PubMed
-
- Castagnola M, Cabras T, Vitali A, Sanna MT, Messana I (2011) Biotechnological implications of the salivary proteome. Trends Biotechnol 29: 409–418. - PubMed
-
- Oppenheim FG, Salih E, Siqueira WL, Zhang W, Helmerhorst EJ (2007) Salivary proteome and its genetic polymorphisms. Ann NY Acad Sci 1098: 22–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

