Role of MTA2 in human cancer
- PMID: 25394532
- PMCID: PMC4425804
- DOI: 10.1007/s10555-014-9518-0
Role of MTA2 in human cancer
Abstract
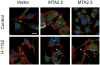
Metastasis is the ultimate cause of death for most cancer patients. While many mechanisms have been delineated for regulation of growth and tumor initiation of the primary tumor, very little is known about the process of metastasis. Metastasis requires dynamic alteration of cellular processes in order for cells to disseminate from the primary tumor to distant sites. These alterations often involve dramatic changes in the regulation of cytoskeletal and cell-environment interactions. Furthermore, controlled refinement of these interactions requires feedback to regulatory networks in the nucleus. MTA2 is a member of the metastasis tumor-associated family of transcriptional regulators and is a central component of the nucleosome remodeling and histone deacetylation complex. MTA2 acts as a central hub for cytoskeletal organization and transcription and provides a link between nuclear and cytoskeletal organization. We will focus on MTA2 in this chapter, especially its role in breast cancer metastasis.
Figures


References
-
- Rigbolt KTG, Prokhorova TA, Akimov V, Henningsen J, Johansen PT, Kratchmarova I, et al. Blagoev B. System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Science signaling. 2011;4(164):rs3. doi: 10.1126/scisignal.2001570. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

