Trends in Kaposi's sarcoma-associated Herpesvirus antibodies prior to the development of HIV-associated Kaposi's sarcoma: a nested case-control study
- PMID: 25395177
- PMCID: PMC4529666
- DOI: 10.1002/ijc.29329
Trends in Kaposi's sarcoma-associated Herpesvirus antibodies prior to the development of HIV-associated Kaposi's sarcoma: a nested case-control study
Abstract
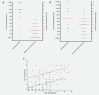
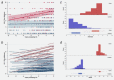
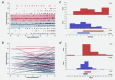
HIV-associated Kaposi's sarcoma (KS) is a public health challenge in sub-Saharan Africa since both the causative agent, Kaposi's sarcoma associated-herpesvirus (KSHV), and the major risk factor, HIV, are prevalent. In a nested case-control study within a long-standing clinical cohort in rural Uganda, we used stored sera to examine the evolution of antibody titres against the KSHV antigens K8.1 and latency-associated nuclear antigen (LANA) among 30 HIV-infected subjects who subsequently developed HIV-related KS (cases) and among 108 matched HIV/KSHV coinfected controls who did not develop KS. Throughout the 6 years prior to diagnosis, antibody titres to K8.1 and LANA were significantly higher among cases than controls (p < 0.0001), and titres increased prior to diagnosis in the cases. K8.1 titres differed more between KS cases and controls, compared to LANA titres. These differences in titre between cases and controls suggest a role for lytic viral replication in the pathogenesis of HIV-related KS in this setting.
Keywords: AIDS; HIV; HIV-associated Kaposi's sarcoma; Kaposi's sarcoma associated-herpesvirus; sub-Saharan Africa.
© 2014 The Authors. Published by Wiley Periodicals, Inc. on behalf of UICC.
Figures
References
-
- Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. - PubMed
-
- Sitas F, Carrara H, Beral V, et al. Antibodies against human herpesvirus 8 in black South African patients with cancer. New Engl J Med. 1999;340:1863–71. - PubMed
-
- Grulich AE, Olsen SJ, Luo K, et al. Kaposi's sarcoma-associated herpesvirus: a sexually transmissible infection? J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:387–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

