Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution
- PMID: 25395301
- PMCID: PMC4304422
- DOI: 10.1189/jlb.1HI0314-154R
Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution
Abstract
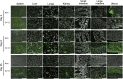
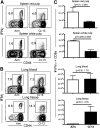
Vaccines are desired that maintain abundant memory T cells at nonlymphoid sites of microbial exposure, where they may be anatomically positioned for immediate pathogen interception. Here, we test the impact of antigen persistence on mouse CD8 and CD4 T cell distribution and differentiation by comparing responses to infections with different strains of LCMV that cause either acute or chronic infections. We used in vivo labeling techniques that discriminate between T cells present within tissues and abundant populations that fail to be removed from vascular compartments, despite perfusion. LCMV persistence caused up to ∼30-fold more virus-specific CD8 T cells to distribute to the lung compared with acute infection. Persistent infection also maintained mucosal-homing α4β7 integrin expression, higher granzyme B expression, alterations in the expression of the TRM markers CD69 and CD103, and greater accumulation of virus-specific CD8 T cells in the large intestine, liver, kidney, and female reproductive tract. Persistent infection also increased LCMV-specific CD4 T cell quantity in mucosal tissues and induced maintenance of CXCR4, an HIV coreceptor. This study clarifies the relationship between viral persistence and CD4 and CD8 T cell distribution and mucosal phenotype, indicating that chronic LCMV infection magnifies T cell migration to nonlymphoid tissues.
Keywords: CD8 T cells; homing.
© Society for Leukocyte Biology.
Figures




Comment in
-
Resident good? Persistent infection increases the number of potentially protective T cells localized in peripheral tissue.J Leukoc Biol. 2015 Feb;97(2):211-3. doi: 10.1189/jlb.1CE0914-422R. J Leukoc Biol. 2015. PMID: 25649788 No abstract available.
References
-
- Mueller S. N., Gebhardt T., Carbone F. R., Heath W. R. (2013) Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161. - PubMed
-
- Kohlmeier J. E., Woodland D. L. (2009) Immunity to respiratory viruses. Annu. Rev. Immunol. 27, 61–82. - PubMed
-
- Gebhardt T., Wakim L. M., Eidsmo L., Reading P. C., Heath W. R., Carbone F. R. (2009) Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

