Amyloidogenic and non-amyloidogenic transthyretin variants interact differently with human cardiomyocytes: insights into early events of non-fibrillar tissue damage
- PMID: 25395306
- PMCID: PMC4293901
- DOI: 10.1042/BSR20140155
Amyloidogenic and non-amyloidogenic transthyretin variants interact differently with human cardiomyocytes: insights into early events of non-fibrillar tissue damage
Abstract
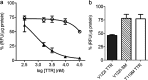
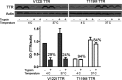
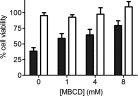
TTR (transthyretin) amyloidoses are diseases characterized by the aggregation and extracellular deposition of the normally soluble plasma protein TTR. Ex vivo and tissue culture studies suggest that tissue damage precedes TTR fibril deposition, indicating that early events in the amyloidogenic cascade have an impact on disease development. We used a human cardiomyocyte tissue culture model system to define these events. We previously described that the amyloidogenic V122I TTR variant is cytotoxic to human cardiac cells, whereas the naturally occurring, stable and non-amyloidogenic T119M TTR variant is not. We show that most of the V122I TTR interacting with the cells is extracellular and this interaction is mediated by a membrane protein(s). In contrast, most of the non-amyloidogenic T119M TTR associated with the cells is intracellular where it undergoes lysosomal degradation. The TTR internalization process is highly dependent on membrane cholesterol content. Using a fluorescent labelled V122I TTR variant that has the same aggregation and cytotoxic potential as the native V122I TTR, we determined that its association with human cardiomyocytes is saturable with a KD near 650 nM. Only amyloidogenic V122I TTR compete with fluorescent V122I for cell-binding sites. Finally, incubation of the human cardiomyocytes with V122I TTR but not with T119M TTR, generates superoxide species and activates caspase 3/7. In summary, our results show that the interaction of the amyloidogenic V122I TTR is distinct from that of a non-amyloidogenic TTR variant and is characterized by its retention at the cell membrane, where it initiates the cytotoxic cascade.
Figures






Similar articles
-
Age-related oxidative modifications of transthyretin modulate its amyloidogenicity.Biochemistry. 2013 Mar 19;52(11):1913-26. doi: 10.1021/bi301313b. Epub 2013 Mar 4. Biochemistry. 2013. PMID: 23414091 Free PMC article.
-
Enhanced transthyretin tetramer stability following expression of an amyloid disease transsuppressor variant in mammalian cells.J Gene Med. 2009 Feb;11(2):103-11. doi: 10.1002/jgm.1276. J Gene Med. 2009. PMID: 19065606 Free PMC article.
-
AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin.Proc Natl Acad Sci U S A. 2013 Jun 11;110(24):9992-7. doi: 10.1073/pnas.1300761110. Epub 2013 May 28. Proc Natl Acad Sci U S A. 2013. PMID: 23716704 Free PMC article.
-
Transthyretin mutagenesis: impact on amyloidogenesis and disease.Crit Rev Clin Lab Sci. 2024 Nov;61(7):616-640. doi: 10.1080/10408363.2024.2350379. Epub 2024 Jun 7. Crit Rev Clin Lab Sci. 2024. PMID: 38850014 Review.
-
Diagnostic and Treatment Approaches Involving Transthyretin in Amyloidogenic Diseases.Int J Mol Sci. 2019 Jun 18;20(12):2982. doi: 10.3390/ijms20122982. Int J Mol Sci. 2019. PMID: 31216785 Free PMC article. Review.
Cited by
-
Echocardiographic risk stratification in light chain and transthyretin amyloidosis: a meta-analysis.Eur Heart J Open. 2025 Aug 22;5(4):oeaf078. doi: 10.1093/ehjopen/oeaf078. eCollection 2025 Jul. Eur Heart J Open. 2025. PMID: 40852192 Free PMC article.
-
A FTIR microspectroscopy study of the structural and biochemical perturbations induced by natively folded and aggregated transthyretin in HL-1 cardiomyocytes.Sci Rep. 2018 Aug 21;8(1):12508. doi: 10.1038/s41598-018-30995-5. Sci Rep. 2018. PMID: 30131519 Free PMC article.
-
Structural Stabilization of Human Transthyretin by Centella asiatica (L.) Urban Extract: Implications for TTR Amyloidosis.Biomolecules. 2019 Mar 29;9(4):128. doi: 10.3390/biom9040128. Biomolecules. 2019. PMID: 30934952 Free PMC article.
-
Molecular Mechanisms of Cardiac Amyloidosis.Int J Mol Sci. 2021 Dec 21;23(1):25. doi: 10.3390/ijms23010025. Int J Mol Sci. 2021. PMID: 35008444 Free PMC article. Review.
-
Targeted treatments of AL and ATTR amyloidosis.Heart Fail Rev. 2022 Sep;27(5):1587-1603. doi: 10.1007/s10741-021-10180-z. Epub 2021 Nov 16. Heart Fail Rev. 2022. PMID: 34783948 Review.
References
-
- Buxbaum J.N. In: In Rheumatology. Hochberg M.C., Silman A.J., Smolen J.S., Weinblatt M.E., Weisman M.H., editors. London: Elsevier; 2008. pp. 1671–1681.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

