DNA recombination. Recombination initiation maps of individual human genomes
- PMID: 25395542
- PMCID: PMC5588152
- DOI: 10.1126/science.1256442
DNA recombination. Recombination initiation maps of individual human genomes
Erratum in
-
Erratum for the Research Article "Recombination initiation maps of individual human genomes" by F. Pratto, K. Brick, P. Khil, F. Smagulova, G. V. Petukhova, R. D. Camerini-Otero.Science. 2018 Oct 19;362(6412):eaav6294. doi: 10.1126/science.aav6294. Science. 2018. PMID: 30337382 No abstract available.
Abstract
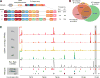
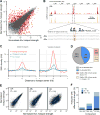
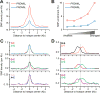
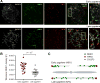
DNA double-strand breaks (DSBs) are introduced in meiosis to initiate recombination and generate crossovers, the reciprocal exchanges of genetic material between parental chromosomes. Here, we present high-resolution maps of meiotic DSBs in individual human genomes. Comparing DSB maps between individuals shows that along with DNA binding by PRDM9, additional factors may dictate the efficiency of DSB formation. We find evidence for both GC-biased gene conversion and mutagenesis around meiotic DSB hotspots, while frequent colocalization of DSB hotspots with chromosome rearrangement breakpoints implicates the aberrant repair of meiotic DSBs in genomic disorders. Furthermore, our data indicate that DSB frequency is a major determinant of crossover rate. These maps provide new insights into the regulation of meiotic recombination and the impact of meiotic recombination on genome function.
Copyright © 2014, American Association for the Advancement of Science.
Figures



Comment in
-
Human genetics. Hidden features of human hotspots.Science. 2014 Nov 14;346(6211):808-9. doi: 10.1126/science.aaa0612. Science. 2014. PMID: 25395519 No abstract available.
References
-
- Baudat F, Imai Y, de Massy B. Meiotic recombination in mammals: localization and regulation. Nat Rev Genet. 2013;14:794–806. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

