Generation of rodent and human osteoblasts
- PMID: 25396049
- PMCID: PMC4230189
- DOI: 10.1038/bonekey.2014.80
Generation of rodent and human osteoblasts
Abstract
This paper describes the isolation, culture and staining of primary osteoblasts from neonatal rodents and human samples. The calvaria and long-bone assays allow direct measurement of bone matrix deposition and mineralisation, as well as producing osteoblasts at defined stages of differentiation for molecular and histological analysis. Culture of human osteoblasts enables cell function to be investigated in targeted patient groups. The described methods will provide a step-by-step guide of what to expect at each stage of the culture and highlight the varied tissue culture conditions required to successfully grow osteoblasts from different sources. A special focus of this paper is the methods used for analysis of bone mineralisation and how to ensure that nonspecific mineral deposition or staining is not quantified.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





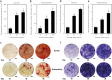


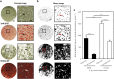
References
-
- Orriss IR, Taylor SE, Arnett TR. Rat osteoblast cultures. Methods Mol Biol 2012;816:31–41. - PubMed
-
- Dillon JP, Waring-Green VJ, Taylor AM, Wilson PJ, Birch M, Gartland A et al. Primary human osteoblast cultures. Methods Mol Biol 2012;816:3–18. - PubMed
-
- Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol 2012;816:19–29. - PubMed
-
- Taylor SEB, Key ML, Lander M, Orriss IR, Patel JJ, Arnett TR. A novel method for the isolation and culture of rat long bone osteoblasts. Bone 2009;44:S318–S319.
-
- Orriss IR, Utting JC, Brandao-Burch A, Colston K, Grubb BR, Burnstock G et al. Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology 2007;148:4208–4216. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
