Small-molecule inhibitors of the MDM2-p53 protein-protein interaction (MDM2 Inhibitors) in clinical trials for cancer treatment
- PMID: 25396320
- PMCID: PMC4329994
- DOI: 10.1021/jm501092z
Small-molecule inhibitors of the MDM2-p53 protein-protein interaction (MDM2 Inhibitors) in clinical trials for cancer treatment
Abstract
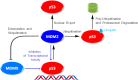
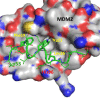
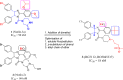
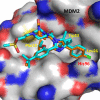
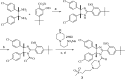
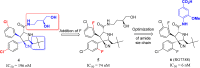
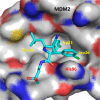
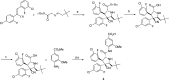
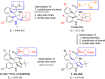
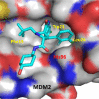
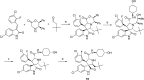
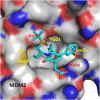
Design of small-molecule inhibitors (MDM2 inhibitors) to block the MDM2-p53 protein-protein interaction has been pursued as a new cancer therapeutic strategy. In recent years, potent, selective, and efficacious MDM2 inhibitors have been successfully obtained and seven such compounds have been advanced into early phase clinical trials for the treatment of human cancers. Here, we review the design, synthesis, properties, preclinical, and clinical studies of these clinical-stage MDM2 inhibitors.
Figures



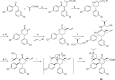
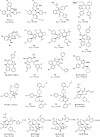
References
-
- Vousden K. H.; Lu X. Live or let die: the cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. - PubMed
-
- Stiewe T. The p53 family in differentiation and tumorigenesis. Nat. Rev. Cancer 2007, 7, 165–168. - PubMed
-
- Toledo F.; Wahl G. M. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat. Rev. Cancer 2006, 6, 909–923. - PubMed
-
- Brown C. J.; Lain S.; Verma C. S.; Fersht A. R.; Lane D. P. Awakening guardian angels: drugging the p53 pathway. Nat. Rev. Cancer 2009, 9, 862–873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Research Materials
Miscellaneous

