Detection theory in identification of RNA-DNA sequence differences using RNA-sequencing
- PMID: 25396741
- PMCID: PMC4232354
- DOI: 10.1371/journal.pone.0112040
Detection theory in identification of RNA-DNA sequence differences using RNA-sequencing
Abstract
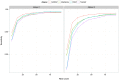
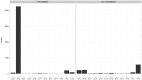
Advances in sequencing technology have allowed for detailed analyses of the transcriptome at single-nucleotide resolution, facilitating the study of RNA editing or sequence differences between RNA and DNA genome-wide. In humans, two types of post-transcriptional RNA editing processes are known to occur: A-to-I deamination by ADAR and C-to-U deamination by APOBEC1. In addition to these sequence differences, researchers have reported the existence of all 12 types of RNA-DNA sequence differences (RDDs); however, the validity of these claims is debated, as many studies claim that technical artifacts account for the majority of these non-canonical sequence differences. In this study, we used a detection theory approach to evaluate the performance of RNA-Sequencing (RNA-Seq) and associated aligners in accurately identifying RNA-DNA sequence differences. By generating simulated RNA-Seq datasets containing RDDs, we assessed the effect of alignment artifacts and sequencing error on the sensitivity and false discovery rate of RDD detection. Overall, we found that even in the presence of sequencing errors, false negative and false discovery rates of RDD detection can be contained below 10% with relatively lenient thresholds. We also assessed the ability of various filters to target false positive RDDs and found them to be effective in discriminating between true and false positives. Lastly, we used the optimal thresholds we identified from our simulated analyses to identify RDDs in a human lymphoblastoid cell line. We found approximately 6,000 RDDs, the majority of which are A-to-G edits and likely to be mediated by ADAR. Moreover, we found the majority of non A-to-G RDDs to be associated with poorer alignments and conclude from these results that the evidence for widespread non-canonical RDDs in humans is weak. Overall, we found RNA-Seq to be a powerful technique for surveying RDDs genome-wide when coupled with the appropriate thresholds and filters.
Conflict of interest statement
Figures



References
-
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 40: 1413–1415. - PubMed
-
- Peng Z, Cheng Y, Tan BC, Kang L, Tian Z, et al. (2012) Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol 30: 253–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

