Prediction of metabolic flux distribution from gene expression data based on the flux minimization principle
- PMID: 25397773
- PMCID: PMC4232356
- DOI: 10.1371/journal.pone.0112524
Prediction of metabolic flux distribution from gene expression data based on the flux minimization principle
Abstract
Prediction of possible flux distributions in a metabolic network provides detailed phenotypic information that links metabolism to cellular physiology. To estimate metabolic steady-state fluxes, the most common approach is to solve a set of macroscopic mass balance equations subjected to stoichiometric constraints while attempting to optimize an assumed optimal objective function. This assumption is justifiable in specific cases but may be invalid when tested across different conditions, cell populations, or other organisms. With an aim to providing a more consistent and reliable prediction of flux distributions over a wide range of conditions, in this article we propose a framework that uses the flux minimization principle to predict active metabolic pathways from mRNA expression data. The proposed algorithm minimizes a weighted sum of flux magnitudes, while biomass production can be bounded to fit an ample range from very low to very high values according to the analyzed context. We have formulated the flux weights as a function of the corresponding enzyme reaction's gene expression value, enabling the creation of context-specific fluxes based on a generic metabolic network. In case studies of wild-type Saccharomyces cerevisiae, and wild-type and mutant Escherichia coli strains, our method achieved high prediction accuracy, as gauged by correlation coefficients and sums of squared error, with respect to the experimentally measured values. In contrast to other approaches, our method was able to provide quantitative predictions for both model organisms under a variety of conditions. Our approach requires no prior knowledge or assumption of a context-specific metabolic functionality and does not require trial-and-error parameter adjustments. Thus, our framework is of general applicability for modeling the transcription-dependent metabolism of bacteria and yeasts.
Conflict of interest statement
Figures
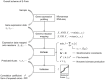
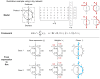
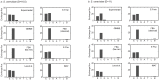
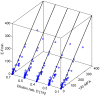
References
-
- Matsushika A, Inoue H, Kodaki T, Sawayama S (2009) Ethanol production from xylose in engineered Saccharomyces cerevisiae strains: current state and perspectives. Appl Microbiol Biotechnol 84: 37–53. - PubMed
-
- Kim HU, Sohn SB, Lee SY (2012) Metabolic network modeling and simulation for drug targeting and discovery. Biotechnol J 7: 330–342. - PubMed
-
- Chiaradonna F, Moresco RM, Airoldi C, Gaglio D, Palorini R, et al. (2012) From cancer metabolism to new biomarkers and drug targets. Biotechnol Adv 30: 30–51. - PubMed
-
- Zamboni N, Fendt SM, Ruhl M, Sauer U (2009) 13C-based metabolic flux analysis. Nat Protoc 4: 878–892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

