Catalytic mechanisms and regulation of protein kinases
- PMID: 25399640
- PMCID: PMC4373616
- DOI: 10.1016/B978-0-12-397918-6.00001-X
Catalytic mechanisms and regulation of protein kinases
Abstract
Protein kinases transfer a phosphoryl group from ATP onto target proteins and play a critical role in signal transduction and other cellular processes. Here, we review the kinase kinetic and chemical mechanisms and their application in understanding kinase structure and function. Aberrant kinase activity has been implicated in many human diseases, in particular cancer. We highlight applications of technologies and concepts derived from kinase mechanistic studies that have helped illuminate how kinases are regulated and contribute to pathophysiology.
Keywords: B-Raf; Bisubstrate analog; Chemical rescue; EGFR; Erlotinib; Inhibitor; Lapatinib; Src; Transition state.
Figures
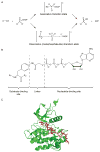
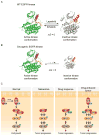
References
-
- Ablooglu AJ, Frankel M, Rusinova E, Ross JB, Kohanski RA. Multiple activation loop conformations and their regulatory properties in the insulin receptor’s kinase domain. Journal of Biological Chemistry. 2001;276:46933–46940. - PubMed
-
- Ablooglu AJ, Till JH, Kim K, Parang K, Cole PA, Hubbard SR, et al. Probing the catalytic mechanism of the insulin receptor kinase with a tetrafluorotyrosine-containing peptide substrate. Journal of Biological Chemistry. 2000;275:30394–30398. - PubMed
-
- Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chemical Reviews. 2001;101:2271–2290. - PubMed
-
- Adams JA, Taylor SS. Energetic limits of phosphotransfer in the catalytic subunit of cAMP-dependent protein kinase as measured by viscosity experiments. Biochemistry. 1992;31:8516–8522. - PubMed
-
- Admiraal SJ, Herschlag D. Mapping the transition state for ATP hydrolysis: Implications for enzymatic catalysis. Chemistry & Biology. 1995;2:729–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

