Probucol suppresses human glioma cell proliferation in vitro via ROS production and LKB1-AMPK activation
- PMID: 25399650
- PMCID: PMC4261125
- DOI: 10.1038/aps.2014.88
Probucol suppresses human glioma cell proliferation in vitro via ROS production and LKB1-AMPK activation
Retraction in
-
Retraction: Probucol suppresses human glioma cell proliferation in vitro via ROS production and LKB1-AMPK activation.Acta Pharmacol Sin. 2018 Feb;39(2):328. doi: 10.1038/aps.2018.2. Acta Pharmacol Sin. 2018. PMID: 29388569 Free PMC article. No abstract available.
Abstract
Aim: Probucol, an anti-hyperlipidemic drug, has been reported to exert antitumor activities at various stages of tumor initiation, promotion and progression. In this study we examined whether the drug affected glioma cell growth in vitro and the underlying mechanisms.
Methods: Human glioma U87 and glioblastoma SF295 cell lines were used. Cell proliferation was accessed using the cell proliferation assay and BrdU incorporation. The phosphorylation of AMPK, liver kinase B1 (LKB1) and p27(Kip1) was detected by Western blot. The activity of 26S proteasome was assessed with an in situ fluorescent substrate. siRNAs were used to suppress the expression of the relevant signaling proteins.
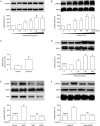
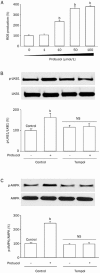
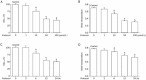
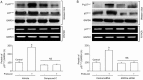
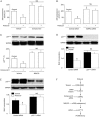
Results: Treatment of U87 glioma cells with probucol (10-100 μmol/L) suppressed the cell proliferation in dose- and time dependent manners. Meanwhile, probucol markedly increased the ROS production, phosphorylation of AMPK at Thr172 and LKB1 at Ser428 in the cells. Furthermore, probucol significantly decreased 26S proteasome activity and increased p27(Kip1) protein level in the cells in an AMPK-dependent manner. Probucol-induced suppression of U87 cell proliferation could be reversed by pretreatment with tempol (a superoxide dismutase mimetic), MG132 (proteasome inhibitor) or compound C (AMPK inhibitor), or by gene silencing of LKB1, AMPK or p27(Kip1). Similar results were observed in probucol-treated SF295 cells.
Conclusion: Probucol suppresses human glioma cell proliferation in vitro via ROS production and LKB1-AMPK activation, which reduces 26S proteasome-dependent degradation of p27(Kip1).
Figures
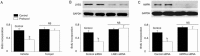

References
-
- Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379:1984–96. - PubMed
-
- Wong ML, Kaye AH, Hovens CM. Targeting malignant glioma survival signalling to improve clinical outcomes. J Clin Neurosci. 2007;14:301–8. - PubMed
-
- Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89:777–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

