Coccolithophore calcification response to past ocean acidification and climate change
- PMID: 25399967
- PMCID: PMC4243242
- DOI: 10.1038/ncomms6363
Coccolithophore calcification response to past ocean acidification and climate change
Abstract
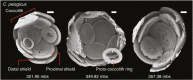
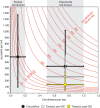
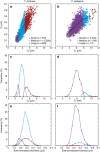
Anthropogenic carbon dioxide emissions are forcing rapid ocean chemistry changes and causing ocean acidification (OA), which is of particular significance for calcifying organisms, including planktonic coccolithophores. Detailed analysis of coccolithophore skeletons enables comparison of calcite production in modern and fossil cells in order to investigate biomineralization response of ancient coccolithophores to climate change. Here we show that the two dominant coccolithophore taxa across the Paleocene-Eocene Thermal Maximum (PETM) OA global warming event (~56 million years ago) exhibited morphological response to environmental change and both showed reduced calcification rates. However, only Coccolithus pelagicus exhibits a transient thinning of coccoliths, immediately before the PETM, that may have been OA-induced. Changing coccolith thickness may affect calcite production more significantly in the dominant modern species Emiliania huxleyi, but, overall, these PETM records indicate that the environmental factors that govern taxonomic composition and growth rate will most strongly influence coccolithophore calcification response to anthropogenic change.
Figures

References
-
- Feely R. A. et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (2004). - PubMed
-
- Doney S. C. et al. Ocean acidification: the other CO2 problem. Annu. Rev. Marine Sci. 1, 169–192 (2009). - PubMed
-
- Fabry V. J. Marine calcifiers in a high-CO2 ocean. Science 320, 1020–1022 (2008). - PubMed
-
- Ridgwell A. et al. From laboratory manipulations to Earth system models: scaling calcification impacts of ocean acidification. Biogeosciences 6, 2611–2623 (2009).
-
- Riebesell U & Tortell P.D. inOcean Acidification (eds Gattuso J. P., Hansson L. 99–121Oxford Univ. Press (2011).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

