Synthesis of Naturally Occurring Tropones and Tropolones
- PMID: 25400298
- PMCID: PMC4228802
- DOI: 10.1016/j.tet.2014.07.065
Synthesis of Naturally Occurring Tropones and Tropolones
Abstract
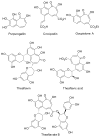
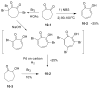
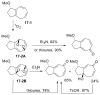
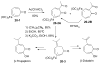
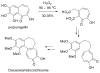
Tropones and tropolones are an important class of seven-membered non-benzenoid aromatic compounds. They can be prepared directly by oxidation of seven-membered rings. They can also be derived from cyclization or cycloaddition of appropriate precursors followed by elimination or rearrangement. This review discusses the types of naturally occurring tropones and tropolones and outlines important methods developed for the synthesis of tropone and tropolone natural products.
Figures
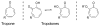











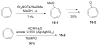



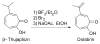








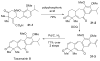







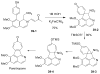









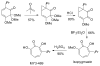
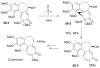




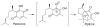








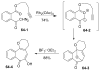


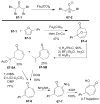

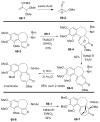

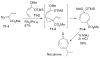


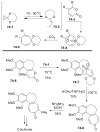



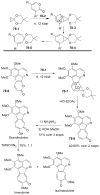


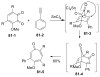
References
-
- Houk KN, Woodward RB. J Am Chem Soc. 1970;92:4145.
-
- Houk KN, Luskus LJ, Bhacca NS. J Am Chem Soc. 1970;92:6392.
-
- Noble WJL, Ojosipe BA. J Am Chem Soc. 1975;97:5939.
-
- Trost BM, Seoane PR. J Am Chem Soc. 1987;109:615.
-
- Machiguchi T, Hasegawa T, Ishii Y, Yamabe S, Minato T. J Am Chem Soc. 1993;115:11536.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
