Diagnostic tests for hepatitis C: recent trends in electrochemical immunosensor and genosensor analysis
- PMID: 25400433
- PMCID: PMC4229514
- DOI: 10.3748/wjg.v20.i42.15476
Diagnostic tests for hepatitis C: recent trends in electrochemical immunosensor and genosensor analysis
Abstract
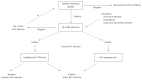
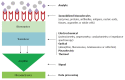
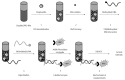
Hepatitis C is a liver disease that is transmitted through contact with the blood of an infected person. An estimated 150 million individuals worldwide have been chronically infected with the hepatitis C virus (HCV). Hepatitis C shows significant genetic variation in the global population, due to the high rate of viral RNA mutation. There are six variants of the virus (HCV genotypes 1, 2, 3, 4, 5, and 6), with 15 recorded subtypes that vary in prevalence across different regions of the world. A variety of devices are used to diagnose hepatitis C, including HCV antibody test, HCV viral load test, HCV genotype test and liver biopsy. Rapid, inexpensive, sensitive, and robust analytical devices are therefore essential for effective diagnosis and monitoring of disease treatment. This review provides an overview of current electrochemical immunosensor and genosensor technologies employed in HCV detection. There are a limited number of publications showing electrochemical biosensors being used for the detection of HCV. Due to their simplicity, specificity, and reliability, electrochemical biosensor devices have potential clinical applications in several viral infections.
Keywords: Diagnostic tests; Electrochemical detection; Genosensors; Hepatitis C virus; Immunosensors.
Figures
Similar articles
-
Hepatitis C virus diagnostics: technology, clinical applications and impacts.Trends Biotechnol. 1997 Feb;15(2):71-6. doi: 10.1016/S0167-7799(97)84206-0. Trends Biotechnol. 1997. PMID: 9081301 Review.
-
Use of virologic assays in the diagnosis and management of hepatitis C virus infection.Clin Liver Dis. 2005 Aug;9(3):371-82, v. doi: 10.1016/j.cld.2005.05.009. Clin Liver Dis. 2005. PMID: 16023971 Review.
-
Expression of the full-length HCV core subgenome from HCV gentoype-1a and genotype-3a and evaluation of the antigenicity of translational products.Eur J Gastroenterol Hepatol. 2013 Jul;25(7):806-13. doi: 10.1097/MEG.0b013e32835eb9b9. Eur J Gastroenterol Hepatol. 2013. PMID: 23442416
-
Diagnostic tests for hepatitis C.Hepatology. 1997 Sep;26(3 Suppl 1):43S-47S. doi: 10.1002/hep.510260708. Hepatology. 1997. PMID: 9305663 Review.
-
Diagnostic tests for hepatitis C.J Hepatol. 1999;31 Suppl 1:71-9. doi: 10.1016/s0168-8278(99)80378-x. J Hepatol. 1999. PMID: 10622564 Review.
Cited by
-
Genosensors as an alternative diagnostic sensing approaches for specific detection of virus species: A review of common techniques and outcomes.Trends Analyt Chem. 2022 Oct;155:116686. doi: 10.1016/j.trac.2022.116686. Epub 2022 May 19. Trends Analyt Chem. 2022. PMID: 35611316 Free PMC article. Review.
-
Nano-engineered screen-printed electrodes: A dynamic tool for detection of viruses.Trends Analyt Chem. 2021 Oct;143:116374. doi: 10.1016/j.trac.2021.116374. Epub 2021 Jun 21. Trends Analyt Chem. 2021. PMID: 34177011 Free PMC article. Review.
-
Update on hepatitis B and C virus diagnosis.World J Virol. 2015 Nov 12;4(4):323-42. doi: 10.5501/wjv.v4.i4.323. World J Virol. 2015. PMID: 26568915 Free PMC article. Review.
-
Contemporary Insights into Hepatitis C Virus: A Comprehensive Review.Microorganisms. 2024 May 21;12(6):1035. doi: 10.3390/microorganisms12061035. Microorganisms. 2024. PMID: 38930417 Free PMC article. Review.
-
Analysis of hepatitis C infection using Raman spectroscopy and proximity based classification in the transformed domain.Biomed Opt Express. 2018 Apr 3;9(5):2041-2055. doi: 10.1364/BOE.9.002041. eCollection 2018 May 1. Biomed Opt Express. 2018. PMID: 29760968 Free PMC article.
References
-
- Tang D, Tang J, Su B, Li Q, Chen G. Electrochemical detection of hepatitis C virus with signal amplification using BamHI endonuclease and horseradish peroxidase-encapsulated nanogold hollow spheres. Chem Commun (Camb) 2011;47:9477–9479. - PubMed
-
- Center for Disease Control and Prevention. Hepatitis C Information for Health Professionals. Accessed in August 25, 2013. Available from: http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm.
-
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10–S15. - PubMed
-
- Torresi J, Johnson D, Wedemeyer H. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J Hepatol. 2011;54:1273–1285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

