IL-13 overexpression in mouse lungs triggers systemic genotoxicity in peripheral blood
- PMID: 25400503
- PMCID: PMC4228791
- DOI: 10.1016/j.mrfmmm.2014.06.007
IL-13 overexpression in mouse lungs triggers systemic genotoxicity in peripheral blood
Abstract
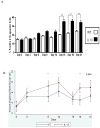
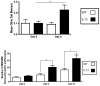
Asthma is a common heterogeneous disease with both genetic and environmental factors that affects millions of individuals worldwide. Activated type 2 helper T cells secrete a panel of cytokines, including IL-13, a central immune regulator of many of the hallmark type 2 disease characteristics found in asthma. IL-13 has been directly implicated as a potent stimulator of asthma induced airway remodeling. Although IL-13 is known to play a major role in the development and persistence of asthma, the complex combination of environmental and genetic origin of the disease obfuscate the solitary role of IL-13 in the disease. We therefore, used a genetically modified mouse model which conditionally overexpresses IL-13 in the lungs to study the independent role of IL-13 in the progression of asthma. Our results demonstrate IL-13 is associated with a systemic induction of genotoxic parameters such as oxidative DNA damage, single and double DNA strand breaks, micronucleus formation, and protein nitration. Furthermore we show that inflammation induced genotoxicity found in asthma extends beyond the primary site of the lung to circulating leukocytes and erythroblasts in the bone marrow eliciting systemic effects driven by IL-13 over-expression.
Conflict of interest statement
Conflicts of interest: The authors declare that there are no conflicts of interest.
Figures





Similar articles
-
Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice.J Immunol. 2005 Aug 15;175(4):2401-7. doi: 10.4049/jimmunol.175.4.2401. J Immunol. 2005. PMID: 16081811
-
Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology.J Clin Invest. 2002 Jan;109(1):29-39. doi: 10.1172/JCI13696. J Clin Invest. 2002. PMID: 11781348 Free PMC article.
-
Differential activation of airway eosinophils induces IL-13-mediated allergic Th2 pulmonary responses in mice.Allergy. 2015 Sep;70(9):1148-59. doi: 10.1111/all.12655. Epub 2015 Jun 14. Allergy. 2015. PMID: 26009788 Free PMC article.
-
Linking surfactant protein SP-D and IL-13: implications in asthma and allergy.Mol Immunol. 2013 May;54(1):98-107. doi: 10.1016/j.molimm.2012.10.039. Epub 2012 Dec 7. Mol Immunol. 2013. PMID: 23220073 Review.
-
New insights into the role of cytokines in asthma.J Clin Pathol. 2001 Aug;54(8):577-89. doi: 10.1136/jcp.54.8.577. J Clin Pathol. 2001. PMID: 11477111 Free PMC article. Review.
Cited by
-
Downregulation of SUMF2 gene in ovalbumin-induced rat model of allergic inflammation.Int J Clin Exp Pathol. 2015 Oct 1;8(10):12053-63. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26722390 Free PMC article.
-
Affect of Early Life Oxygen Exposure on Proper Lung Development and Response to Respiratory Viral Infections.Front Med (Lausanne). 2015 Aug 10;2:55. doi: 10.3389/fmed.2015.00055. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26322310 Free PMC article. Review.
-
Overexpression of miR-155-5p Inhibits the Proliferation and Migration of IL-13-Induced Human Bronchial Smooth Muscle Cells by Suppressing TGF-β-Activated Kinase 1/MAP3K7-Binding Protein 2.Allergy Asthma Immunol Res. 2018 May;10(3):260-267. doi: 10.4168/aair.2018.10.3.260. Allergy Asthma Immunol Res. 2018. PMID: 29676073 Free PMC article.
-
Impaired regenerative capacity contributes to skeletal muscle dysfunction in chronic obstructive pulmonary disease.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C974-C989. doi: 10.1152/ajpcell.00292.2022. Epub 2022 Aug 22. Am J Physiol Cell Physiol. 2022. PMID: 35993519 Free PMC article. Review.
-
IL-13-driven pulmonary emphysema leads to skeletal muscle dysfunction attenuated by endurance exercise.J Appl Physiol (1985). 2020 Jan 1;128(1):134-148. doi: 10.1152/japplphysiol.00627.2019. Epub 2019 Nov 27. J Appl Physiol (1985). 2020. PMID: 31774358 Free PMC article.
References
-
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States,2005-2009. Natl Health Stat Report. 2011:1–14. - PubMed
-
- Miller AL. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern Med Rev. 2001;6:20–47. - PubMed
-
- Romanet-Manent S, Charpin D, Magnan A, Lanteaume A, Vervloet D. Allergic vs nonallergic asthma: what makes the difference? Allergy. 2002;57:607–613. - PubMed
-
- Fireman P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003;24:79–83. - PubMed
-
- Hyde DM, Miller LA, Schelegle ES, et al. Asthma: a comparison of animal models using stereological methods. European Respiratory Review. 2006;15:122–135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

