A review of β-amyloid neuroimaging in Alzheimer's disease
- PMID: 25400539
- PMCID: PMC4215612
- DOI: 10.3389/fnins.2014.00327
A review of β-amyloid neuroimaging in Alzheimer's disease
Abstract
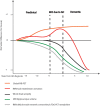
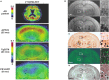
Alzheimer's disease (AD) is the most common cause of dementia worldwide. As advancing age is the greatest risk factor for developing AD, the number of those afflicted is expected to increase markedly with the aging of the world's population. The inability to definitively diagnose AD until autopsy remains an impediment to establishing effective targeted treatments. Neuroimaging has enabled in vivo visualization of pathological changes in the brain associated with the disease, providing a greater understanding of its pathophysiological development and progression. However, neuroimaging biomarkers do not yet offer clear advantages over current clinical diagnostic criteria for them to be accepted into routine clinical use. Nonetheless, current insights from neuroimaging combined with the elucidation of biochemical and molecular processes in AD are informing the ongoing development of new imaging techniques and their application. Much of this research has been greatly assisted by the availability of transgenic mouse models of AD. In this review we summarize the main efforts of neuroimaging in AD in humans and in mouse models, with a specific focus on β-amyloid, and discuss the potential of new applications and novel approaches.
Keywords: Alzheimer's disease; CT; MRI; PET; biomarkers; mouse models; neuroimaging.
Figures
References
-
- Antharam V., Collingwood J. F., Bullivant J. P., Davidson M. R., Chandra S., Mikhaylova A., et al. (2012). High field magnetic resonance microscopy of the human hippocampus in Alzheimer's disease: quantitative imaging and correlation with iron. Neuroimage 59, 1249–1260. 10.1016/j.neuroimage.2011.08.019 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

