Conceptualizing lennox-gastaut syndrome as a secondary network epilepsy
- PMID: 25400619
- PMCID: PMC4214194
- DOI: 10.3389/fneur.2014.00225
Conceptualizing lennox-gastaut syndrome as a secondary network epilepsy
Abstract
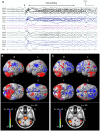
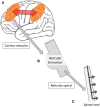
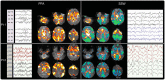
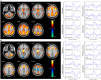
Lennox-Gastaut Syndrome (LGS) is a category of severe, disabling epilepsy, characterized by frequent, treatment-resistant seizures, and cognitive impairment. Electroencephalography (EEG) shows characteristic generalized epileptic activity that is similar in those with lesional, genetic, or unknown causes, suggesting a common underlying mechanism. The condition typically begins in young children, leaving many severely disabled with recurring seizures throughout their adult life. Scalp EEG of the tonic seizures of LGS is characterized by a diffuse high-voltage slow transient evolving into generalized low-voltage fast activity, likely reflecting sustained fast neuronal firing over a wide cortical area. The typical interictal discharges (runs of slow spike-and-wave and bursts of generalized paroxysmal fast activity) also have a "generalized" electrical field, suggesting widespread cortical involvement. Recent brain mapping studies have begun to reveal which cortical and subcortical regions are active during these "generalized" discharges. In this critical review, we examine findings from neuroimaging studies of LGS and place these in the context of the electrical and clinical features of the syndrome. We suggest that LGS can be conceptualized as "secondary network epilepsy," where the epileptic activity is expressed through large-scale brain networks, particularly the attention and default-mode networks. Cortical lesions, when present, appear to chronically interact with these networks to produce network instability rather than triggering each individual epileptic discharge. LGS can be considered as "secondary" network epilepsy because the epileptic manifestations of the disorder reflect the networks being driven, rather than the specific initiating process. In this review, we begin with a summation of the clinical manifestations of LGS and what this has revealed about the underlying etiology of the condition. We then undertake a systematic review of the functional neuroimaging literature in LGS, which leads us to conclude that LGS can best be conceptualized as "secondary network epilepsy."
Keywords: EEG–fMRI; Lennox–Gastaut syndrome; attention network; default-mode network; generalized epilepsy; paroxysmal fast activity; slow spike and wave; tonic seizure.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

