Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery
- PMID: 25400717
- PMCID: PMC4232191
- DOI: 10.2174/1877912311202020082
Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery
Abstract
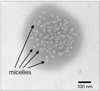
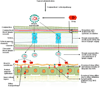
One of the most challenging areas of pharmaceutical research is ocular drug delivery. The unique anatomy and physiology of the eye impedes drug permeation to deeper ocular tissues. Nanosized carrier systems such as nanoparticles, liposomes, suspensions, dendrimers, and nanomicelles are being explored for ocular drug delivery. In this review, we have focused on application of emerging nanomicellar carrier systems in ocular drug delivery. Nanomicelles are nanosized vesicular carriers formed from amphiphilic monomer units. Surfactant and polymeric micellar nanocarriers provide an amenable means to improve drug solubilization, develop clear aqueous formulations and deliver drugs to anterior and posterior ocular tissues. Nanomicelles due to their amphiphilic nature encapsulate hydrophobic drugs and aid in drug delivery. Various methods are employed to develop nanosized micellar formulations depending upon the physicochemical properties of the drug. Nanomicellar carriers appear to be promising vehicles with potential applications in ocular drug delivery. In this review, we attempted to discuss about the progress in ocular drug delivery research using nanomicelles as carriers from the published literature and issued patents. Also, with regards to ocular static and dynamic barriers which prevent drug permeation, a brief discussion about nanomicelles, types of nanomicelles, their methods of preparation and micellar strategy to overcome ocular barriers, delivering therapeutic levels of drugs to anterior and posterior ocular tissues are discussed.
Keywords: Barriers; conjunctival/scleral pathway; drug delivery; nanomicelles; nanotechnology; patents; posterior segment; retina-choroid.
Conflict of interest statement
There is no conflict of interest.
Figures



References
-
- Forrest ML, Won C-Y, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(ε-caprolactone) micelles. J Controlled Release. 2006;110(2):370–377. - PubMed
-
- Loftsson T, Hreinsdottir D. Determination of aqueous solubility by heating and equilibration: A technical note. AAPS Pharm Sci Tech. 2006;7(1):E4. - PubMed
-
- Cunningham MA, Edelman JL, Kaushal S. Intravitreal steroids for macular edema: The past, the present, and the future. Surv Ophthalmol. 2008;53(2):139–149. - PubMed
-
- Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye (Lond) 2008;22(4):590–591. - PubMed
-
- Michael S, Ip M. Intravitreal injection of triamcinolone. An emerging treatment for diabetic macular edema. Diabetes Care. 2004;27(7):1794–1797. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
