Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in cerebellum and brainstem
- PMID: 25400899
- PMCID: PMC4233053
- DOI: 10.1186/2040-2392-5-47
Saccadic eye movement abnormalities in autism spectrum disorder indicate dysfunctions in cerebellum and brainstem
Abstract
Background: Individuals with autism spectrum disorder (ASD) show atypical scan paths during social interaction and when viewing faces, and recent evidence suggests that they also show abnormal saccadic eye movement dynamics and accuracy when viewing less complex and non-social stimuli. Eye movements are a uniquely promising target for studies of ASD as their spatial and temporal characteristics can be measured precisely and the brain circuits supporting them are well-defined. Control of saccade metrics is supported by discrete circuits within the cerebellum and brainstem - two brain regions implicated in magnetic resonance (MR) morphometry and histopathological studies of ASD. The functional integrity of these distinct brain systems can be examined by evaluating different parameters of visually-guided saccades.
Methods: A total of 65 participants with ASD and 43 healthy controls, matched on age (between 6 and 44-years-old), gender and nonverbal IQ made saccades to peripheral targets. To examine the influence of attentional processes, blocked gap and overlap trials were presented. We examined saccade latency, accuracy and dynamics, as well as the trial-to-trial variability of participants' performance.
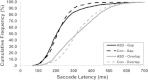
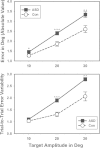
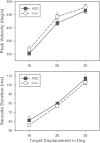
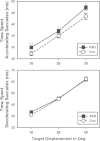
Results: Saccades of individuals with ASD were characterized by reduced accuracy, elevated variability in accuracy across trials, and reduced peak velocity and prolonged duration. In addition, their saccades took longer to accelerate to peak velocity, with no alteration in the duration of saccade deceleration. Gap/overlap effects on saccade latencies were similar across groups, suggesting that visual orienting and attention systems are relatively spared in ASD. Age-related changes did not differ across groups.
Conclusions: Deficits precisely and consistently directing eye movements suggest impairment in the error-reducing function of the cerebellum in ASD. Atypical increases in the duration of movement acceleration combined with lower peak saccade velocities implicate pontine nuclei, specifically suggesting reduced excitatory activity in burst cells that drive saccades relative to inhibitory activity in omnipause cells that maintain stable fixation. Thus, our findings suggest that both cerebellar and brainstem abnormalities contribute to altered sensorimotor control in ASD.
Keywords: Autism spectrum disorder (ASD); Brainstem; Cerebellum; Eye movement; Saccade; Sensorimotor.
Figures

Similar articles
-
Saccadic eye movements in adults with high-functioning autism spectrum disorder.Autism. 2018 Feb;22(2):195-204. doi: 10.1177/1362361316667057. Epub 2016 Nov 14. Autism. 2018. PMID: 29490485
-
Basic oculomotor function is similar in young children with ASD and typically developing controls.Autism Res. 2021 Dec;14(12):2580-2591. doi: 10.1002/aur.2592. Epub 2021 Aug 18. Autism Res. 2021. PMID: 34405961
-
Initial action output and feedback-guided motor behaviors in autism spectrum disorder.Mol Autism. 2021 Jul 10;12(1):52. doi: 10.1186/s13229-021-00452-8. Mol Autism. 2021. PMID: 34246292 Free PMC article.
-
[Neurophysiological studies in autism spectrum disorders--comparison with those in schizophrenia].Seishin Shinkeigaku Zasshi. 2012;114(4):335-48. Seishin Shinkeigaku Zasshi. 2012. PMID: 22712203 Review. Japanese.
-
Cerebro-cerebellar circuits in autism spectrum disorder.Front Neurosci. 2015 Nov 5;9:408. doi: 10.3389/fnins.2015.00408. eCollection 2015. Front Neurosci. 2015. PMID: 26594140 Free PMC article. Review.
Cited by
-
Study of an Extensive Set of Eye Movement Features: Extraction Methods and Statistical Analysis.J Eye Mov Res. 2018 Mar 20;11(1):10.16910/jemr.11.1.3. doi: 10.16910/jemr.11.1.3. J Eye Mov Res. 2018. PMID: 33828682 Free PMC article.
-
Eye-Hand Coordination Patterns of Intermediate and Novice Surgeons in a Simulation-Based Endoscopic Surgery Training Environment.J Eye Mov Res. 2018 Nov 8;11(6):10.16910/jemr.11.6.1. doi: 10.16910/jemr.11.6.1. J Eye Mov Res. 2018. PMID: 33828711 Free PMC article.
-
Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice.Nat Commun. 2016 Sep 1;7:12627. doi: 10.1038/ncomms12627. Nat Commun. 2016. PMID: 27581745 Free PMC article.
-
How Saccade Intrusions Affect Subsequent Motor and Oculomotor Actions.Front Neurosci. 2017 Jan 12;10:608. doi: 10.3389/fnins.2016.00608. eCollection 2016. Front Neurosci. 2017. PMID: 28127274 Free PMC article.
-
Eye movements, sensorimotor adaptation and cerebellar-dependent learning in autism: toward potential biomarkers and subphenotypes.Eur J Neurosci. 2018 Mar;47(6):549-555. doi: 10.1111/ejn.13625. Epub 2017 Jul 12. Eur J Neurosci. 2018. PMID: 28612953 Free PMC article. Review.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Publishing; 2013.
-
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Botteron KN, Dager SR, Estes AM, Evans AC, Gerig G, Hazlett HC, Schultz RT, Styner M, Zwaigenbaum L, Piven J. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170:899–908. doi: 10.1176/appi.ajp.2012.12091150. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

