Recent advances in hydrogen peroxide imaging for biological applications
- PMID: 25400906
- PMCID: PMC4232666
- DOI: 10.1186/2045-3701-4-64
Recent advances in hydrogen peroxide imaging for biological applications
Abstract
Mounting evidence supports the role of hydrogen peroxide (H2O2) in physiological signaling as well as pathological conditions. However, the subtleties of peroxide-mediated signaling are not well understood, in part because the generation, degradation, and diffusion of H2O2 are highly volatile within different cellular compartments. Therefore, the direct measurement of H2O2 in living specimens is critically important. Fluorescent probes that can detect small changes in H2O2 levels within relevant cellular compartments are important tools to study the spatial dynamics of H2O2. To achieve temporal resolution, the probes must also be photostable enough to allow multiple readings over time without loss of signal. Traditional fluorescent redox sensitive probes that have been commonly used for the detection of H2O2 tend to react with a wide variety of reactive oxygen species (ROS) and often suffer from photostablilty issues. Recently, new classes of H2O2 probes have been designed to detect H2O2 with high selectivity. Advances in H2O2 measurement have enabled biomedical scientists to study H2O2 biology at a level of precision previously unachievable. In addition, new imaging techniques such as two-photon microscopy (TPM) have been employed for H2O2 detection, which permit real-time measurements of H2O2 in vivo. This review focuses on recent advances in H2O2 probe development and optical imaging technologies that have been developed for biomedical applications.
Keywords: Chemiluminescence; Fluorescence lifetime imaging microscopy (FLIM); Fluorescent probe; Hydrogen peroxide (H2O2); Molecular imaging; Nanoparticles; Ratiometric imaging; Reactive oxygen species (ROS); Two-photon microscopy.
Figures
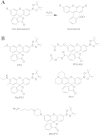
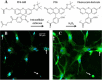
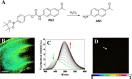
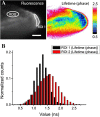
Similar articles
-
Detection of Hydrogen Peroxide with Fluorescent Dyes.Antioxid Redox Signal. 2018 Aug 20;29(6):585-602. doi: 10.1089/ars.2017.7401. Epub 2017 Dec 13. Antioxid Redox Signal. 2018. PMID: 29054131 Review.
-
Recent advances in fluorescent probes for the detection of reactive oxygen species.Anal Bioanal Chem. 2006 Oct;386(3):532-43. doi: 10.1007/s00216-006-0366-9. Epub 2006 May 13. Anal Bioanal Chem. 2006. PMID: 16609844 Review.
-
A Two-Photon Ratiometric Fluorescent Probe for Imaging of Hydrogen Peroxide Levels in Rat Organ Tissues.ChemistryOpen. 2017 Nov 22;7(1):53-56. doi: 10.1002/open.201700155. eCollection 2018 Jan. ChemistryOpen. 2017. PMID: 29318096 Free PMC article.
-
A 3,7-Dihydroxyphenoxazine-based Fluorescent Probe for Selective Detection of Intracellular Hydrogen Peroxide.Chem Asian J. 2016 Mar 18;11(6):818-22. doi: 10.1002/asia.201501304. Epub 2016 Feb 10. Chem Asian J. 2016. PMID: 26807851
-
Mechanisms and Applications of Redox-Sensitive Green Fluorescent Protein-Based Hydrogen Peroxide Probes.Antioxid Redox Signal. 2018 Aug 20;29(6):552-568. doi: 10.1089/ars.2017.7449. Epub 2018 Jan 10. Antioxid Redox Signal. 2018. PMID: 29160083 Review.
Cited by
-
Molecular probes for fluorescence lifetime imaging.Bioconjug Chem. 2015 Jun 17;26(6):963-74. doi: 10.1021/acs.bioconjchem.5b00167. Epub 2015 May 22. Bioconjug Chem. 2015. PMID: 25961514 Free PMC article. Review.
-
Design and Characterization of Hyperpolarized 15N-BBCP as a H2O2-Sensing Probe.ACS Sens. 2022 Oct 28;7(10):2928-2933. doi: 10.1021/acssensors.2c01720. Epub 2022 Oct 18. ACS Sens. 2022. PMID: 36255172 Free PMC article.
-
Assessing the range of enzymatic and oxidative tunability for biosensor design.J Mater Chem B. 2020 Apr 29;8(16):3460-3487. doi: 10.1039/c9tb02666e. J Mater Chem B. 2020. PMID: 32159202 Free PMC article. Review.
-
MACA Fast and Efficient Method for Detecting H2O2 by a Dual-Locked Model Chemosensor.ACS Omega. 2021 May 28;6(23):14819-14823. doi: 10.1021/acsomega.1c00384. eCollection 2021 Jun 15. ACS Omega. 2021. PMID: 34151063 Free PMC article.
-
Solid State Sensors for Hydrogen Peroxide Detection.Biosensors (Basel). 2020 Dec 25;11(1):9. doi: 10.3390/bios11010009. Biosensors (Basel). 2020. PMID: 33375685 Free PMC article. Review.
References
-
- Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, Murphy TH, Guo H, Langley B, Sultana R, Butterfield DA, Santagata S, Alldred MJ, Gazaryan IG, Bell GW, Ginsberg SD, Ratan RR. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A. 2010;107:17385–17390. doi: 10.1073/pnas.1003996107. - DOI - PMC - PubMed
-
- Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

