Relative contribution of IL-1α, IL-1β and TNF to the host response to Mycobacterium tuberculosis and attenuated M. bovis BCG
- PMID: 25400917
- PMCID: PMC4217540
- DOI: 10.1002/iid3.9
Relative contribution of IL-1α, IL-1β and TNF to the host response to Mycobacterium tuberculosis and attenuated M. bovis BCG
Abstract
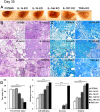
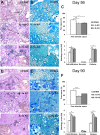
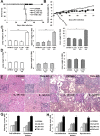
TNF and IL-1 are major mediators involved in severe inflammatory diseases against which therapeutic neutralizing antibodies are developed. However, both TNF and IL-1 receptor pathways are essential for the control of Mycobacterium tuberculosis infection, and it is critical to assess the respective role of IL-1α, IL-1β, and TNF. Using gene-targeted mice we show that absence of both IL-1α and IL-1β recapitulates the uncontrolled M. tuberculosis infection with increased bacterial burden, exacerbated lung inflammation, high IFNγ, reduced IL-23 p19 and rapid death seen in IL-1R1-deficient mice. However, presence of either IL-1α or IL-1β in single-deficient mice is sufficient to control acute M. tuberculosis infection, with restrained bacterial burden and lung pathology, in conditions where TNF deficient mice succumbed within 4 weeks with overwhelming infection. Systemic infection by attenuated M. bovis BCG was controlled in the absence of functional IL-1 pathway, but not in the absence of TNF. Therefore, although both IL-1α and IL-1β are required for a full host response to virulent M. tuberculosis, the presence of either IL-1α or IL-1β allows some control of acute M. tuberculosis infection, and IL-1 pathway is dispensable for controlling M. bovis BCG acute infection. This is in sharp contrast with TNF, which is essential for host response to both attenuated and virulent mycobacteria and may have implications for anti-inflammatory therapy with IL-1β neutralizing antibodies.
Keywords: Host response; IL-1β/IL-1α; M. bovis infection; M. tuberculosis; TNF.
Figures



References
-
- Dye C. Watt CJ, Bleed DM, Hosseini SM. Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. - PubMed
-
- Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 328:856–861. - PubMed
-
- Keane J. TNF-blocking agents and tuberculosis: new drugs illuminate an old topic. Rheumatology (Oxford) 2005;44:714–720. - PubMed
-
- Keane J. Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN. Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 2001;345:1098–1104. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

