Strategies to Detect Hepatic Myofibroblasts in Liver Cirrhosis of Different Etiologies
- PMID: 25401051
- PMCID: PMC4223535
- DOI: 10.1007/s40139-014-0057-8
Strategies to Detect Hepatic Myofibroblasts in Liver Cirrhosis of Different Etiologies
Abstract
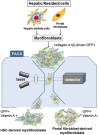
Liver cirrhosis, a late stage of hepatic fibrosis, is an increasing cause of morbidity and mortality worldwide. Hepatic fibrosis is mainly caused by alcoholic or non-alcoholic steatohepatitis, chronic viral hepatitis, or autoimmune and biliary diseases. Myofibroblasts, which are absent from the normal liver, are differentiated from heterogeneous cell populations in response to a liver injury of any etiology and produce the extracellular matrix. Hepatic stellate cells are considered the main source of myofibroblasts. However, the origin of hepatic myofibroblasts remains unresolved, and despite considerable research, only a limited success has been achieved by existing anti-fibrotic therapies. The question remains whether these limitations are caused by lack of attention to the critical targets, the myofibroblasts derived from cells of other mesenchymal origins. Therefore, identifying the origin of myofibroblasts may provide insight into the mechanisms underlying liver fibrosis, and may lead to the development of more effective therapies. This review will examine our current strategies for detecting hepatic myofibroblasts of different origins.
Keywords: Hepatic fibrosis; Hepatic stellate cells; Liver cirrhosis; Myofibroblasts; Portal fibroblasts.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
