Longistatin in tick saliva blocks advanced glycation end-product receptor activation
- PMID: 25401185
- PMCID: PMC4191044
- DOI: 10.1172/jci74917
Longistatin in tick saliva blocks advanced glycation end-product receptor activation
Abstract
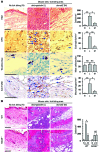
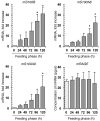
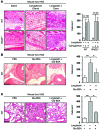
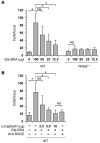
Ticks are notorious hematophagous ectoparasites and vectors of many deadly pathogens. As an effective vector, ticks must break the strong barrier provided by the skin of their host during feeding, and their saliva contains a complex mixture of bioactive molecules that paralyze host defenses. The receptor for advanced glycation end products (RAGE) mediates immune cell activation at inflammatory sites and is constitutively and highly expressed in skin. Here, we demonstrate that longistatin secreted with saliva of the tick Haemaphysalis longicornis binds RAGE and modulates the host immune response. Similar to other RAGE ligands, longistatin specifically bound the RAGE V domain, and stimulated cultured HUVECs adhered to a longistatin-coated surface; this binding was dramatically inhibited by soluble RAGE or RAGE siRNA. Treatment of HUVECs with longistatin prior to stimulation substantially attenuated cellular oxidative stress and prevented NF-κB translocation, thereby reducing adhesion molecule and cytokine production. Recombinant longistatin inhibited RAGE-mediated migration of mouse peritoneal resident cells (mPRCs) and ameliorated inflammation in mouse footpad edema and pneumonia models. Importantly, tick bite upregulated RAGE ligands in skin, and endogenous longistatin attenuated RAGE-mediated inflammation during tick feeding. Our results suggest that longistatin is a RAGE antagonist that suppresses tick bite-associated inflammation, allowing successful blood-meal acquisition from hosts.
Figures





Similar articles
-
Longistatin in tick-saliva targets RAGE.Oncotarget. 2015 Nov 3;6(34):35133-4. doi: 10.18632/oncotarget.6032. Oncotarget. 2015. PMID: 26577647 Free PMC article. No abstract available.
-
Longistatin, a novel EF-hand protein from the ixodid tick Haemaphysalis longicornis, is required for acquisition of host blood-meals.Int J Parasitol. 2010 May;40(6):721-9. doi: 10.1016/j.ijpara.2009.11.004. Epub 2009 Dec 5. Int J Parasitol. 2010. PMID: 19968997
-
Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks.PLoS Pathog. 2011 Mar;7(3):e1001312. doi: 10.1371/journal.ppat.1001312. Epub 2011 Mar 10. PLoS Pathog. 2011. PMID: 21423674 Free PMC article.
-
Receptor for Advanced Glycation End Product (RAGE) Modulates Inflammation During Feeding of the Hard Tick, Haemaphysalis longicornis in Mice.Parasite Immunol. 2024 Jun;46(6):e13039. doi: 10.1111/pim.13039. Parasite Immunol. 2024. PMID: 38838041 Review.
-
[Role of the receptor for advanced glycation end products (RAGE) in inflammation].Invest Clin. 2010 Jun;51(2):257-68. Invest Clin. 2010. PMID: 20928981 Review. Spanish.
Cited by
-
Ambivalent Roles of Oxidative Stress in Triangular Relationships among Arthropod Vectors, Pathogens and Hosts.Antioxidants (Basel). 2022 Jun 25;11(7):1254. doi: 10.3390/antiox11071254. Antioxidants (Basel). 2022. PMID: 35883744 Free PMC article. Review.
-
Research note: Mite infestations in non-descriptive indigenous chickens in Bangladesh: Present status and pathology.Poult Sci. 2025 Mar;104(3):104889. doi: 10.1016/j.psj.2025.104889. Epub 2025 Feb 6. Poult Sci. 2025. PMID: 39952144 Free PMC article.
-
Host Immune Responses to Salivary Components - A Critical Facet of Tick-Host Interactions.Front Cell Infect Microbiol. 2022 Mar 16;12:809052. doi: 10.3389/fcimb.2022.809052. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35372098 Free PMC article. Review.
-
Chemical Equilibrium at the Tick-Host Feeding Interface:A Critical Examination of Biological Relevance in Hematophagous Behavior.Front Physiol. 2019 May 1;10:530. doi: 10.3389/fphys.2019.00530. eCollection 2019. Front Physiol. 2019. PMID: 31118903 Free PMC article. Review.
-
Binding Molecules in Tick Saliva for Targeting Host Cytokines, Chemokines, and Beyond.Biomolecules. 2024 Dec 21;14(12):1647. doi: 10.3390/biom14121647. Biomolecules. 2024. PMID: 39766354 Free PMC article. Review.
References
-
- Sonenshine DE. Biology Of Ticks. Vol 1, New York, New York, USA: Oxford University Press; 1991.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

