A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy
- PMID: 25401298
- PMCID: PMC4281260
- DOI: 10.1038/ng.3144
A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy
Abstract
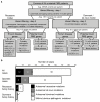
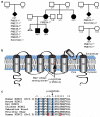
Progressive myoclonus epilepsies (PMEs) are a group of rare, inherited disorders manifesting with action myoclonus, tonic-clonic seizures and ataxia. We sequenced the exomes of 84 unrelated individuals with PME of unknown cause and molecularly solved 26 cases (31%). Remarkably, a recurrent de novo mutation, c.959G>A (p.Arg320His), in KCNC1 was identified as a new major cause for PME. Eleven unrelated exome-sequenced (13%) and two affected individuals in a secondary cohort (7%) had this mutation. KCNC1 encodes KV3.1, a subunit of the KV3 voltage-gated potassium ion channels, which are major determinants of high-frequency neuronal firing. Functional analysis of the Arg320His mutant channel showed a dominant-negative loss-of-function effect. Ten cases had pathogenic mutations in known PME-associated genes (NEU1, NHLRC1, AFG3L2, EPM2A, CLN6 and SERPINI1). Identification of mutations in PRNP, SACS and TBC1D24 expand their phenotypic spectra to PME. These findings provide insights into the molecular genetic basis of PME and show the role of de novo mutations in this disease entity.
Figures

References
-
- Berkovic SF, Andermann F, Carpenter S, Wolfe LS. Progressive Myoclonus Epilepsies: Specific Causes and Diagnosis. N. Engl. J. Med. 1986;315:296–305. - PubMed
-
- Shahwan A, Farrell M, Delanty N. Progressive myoclonic epilepsies: a review of genetic and therapeutic aspects. Lancet Neurol. 2005;4:239–248. - PubMed
-
- Pennacchio LA, et al. Mutations in the Gene Encoding Cystatin B in Progressive Myoclonus Epilepsy (EPM1) Science. 1996;271:1731–1734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

