Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-κB dependent pathway
- PMID: 25401327
- PMCID: PMC4234737
- DOI: 10.1371/journal.pone.0112796
Mycoplasma gallisepticum lipid associated membrane proteins up-regulate inflammatory genes in chicken tracheal epithelial cells via TLR-2 ligation through an NF-κB dependent pathway
Abstract
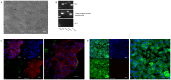
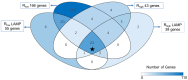
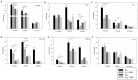
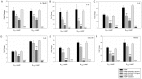
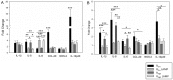
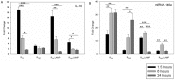
Mycoplasma gallisepticum-mediated respiratory inflammation in chickens is associated with accumulation of leukocytes in the tracheal submucosa. However the molecular mechanisms underpinning these changes have not been well described. We hypothesized that the initial inflammatory events are initiated upon ligation of mycoplasma lipid associated membrane proteins (LAMP) to TLRs expressed on chicken tracheal epithelial cells (TEC). To test this hypothesis, live bacteria or LAMPs isolated from a virulent (R(low)) or a non-virulent (R(high)) strain were incubated with primary TECs or chicken tracheae ex vivo. Microarray analysis identified up-regulation of several inflammatory and chemokine genes in TECs as early as 1.5 hours post-exposure. Kinetic analysis using RT-qPCR identified the peak of expression for most genes to be at either 1.5 or 6 hours. Ex-vivo exposure also showed up-regulation of inflammatory genes in epithelial cells by 1.5 hours. Among the commonly up-regulated genes were IL-1β, IL-6, IL-8, IL-12p40, CCL-20, and NOS-2, all of which are important immune-modulators and/or chemo-attractants of leukocytes. While these inflammatory genes were up-regulated in all four treatment groups, R(low) exposed epithelial cells both in vitro and ex vivo showed the most dramatic up-regulation, inducing over 100 unique genes by 5-fold or more in TECs. Upon addition of a TLR-2 inhibitor, LAMP-mediated gene expression of IL-1β and CCL-20 was reduced by almost 5-fold while expression of IL-12p40, IL-6, IL-8 and NOS-2 mRNA was reduced by about 2-3 fold. Conversely, an NF-κB inhibitor abrogated the response entirely for all six genes. miRNA-146a, a negative regulator of TLR-2 signaling, was up-regulated in TECs in response to either R(low) or R(high) exposure. Taken together we conclude that LAMPs isolated from both R(high) and R(low) induced rapid, TLR-2 dependent but transient up-regulation of inflammatory genes in primary TECs through an NF-κB dependent pathway.
Conflict of interest statement
Figures
Similar articles
-
TLR2/MyD88/NF-κB signaling pathway regulates IL-1β production in DF-1 cells exposed to Mycoplasma gallisepticum LAMPs.Microb Pathog. 2018 Apr;117:225-231. doi: 10.1016/j.micpath.2018.02.037. Epub 2018 Feb 19. Microb Pathog. 2018. PMID: 29471139
-
Interaction of Mycoplasma gallisepticum with Chicken Tracheal Epithelial Cells Contributes to Macrophage Chemotaxis and Activation.Infect Immun. 2015 Nov 2;84(1):266-74. doi: 10.1128/IAI.01113-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26527215 Free PMC article.
-
Mycoplasma bovis-derived lipid-associated membrane proteins activate IL-1β production through the NF-κB pathway via toll-like receptor 2 and MyD88.Dev Comp Immunol. 2016 Feb;55:111-8. doi: 10.1016/j.dci.2015.10.017. Epub 2015 Oct 23. Dev Comp Immunol. 2016. PMID: 26499291
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
gga-miR-99a targets SMARCA5 to regulate Mycoplasma gallisepticum (HS strain) infection by depressing cell proliferation in chicken.Gene. 2017 Sep 5;627:239-247. doi: 10.1016/j.gene.2017.06.039. Epub 2017 Jun 23. Gene. 2017. PMID: 28652181 Review.
Cited by
-
Baicalin ameliorates oxidative stress and apoptosis by restoring mitochondrial dynamics in the spleen of chickens via the opposite modulation of NF-κB and Nrf2/HO-1 signaling pathway during Mycoplasma gallisepticum infection.Poult Sci. 2019 Dec 1;98(12):6296-6310. doi: 10.3382/ps/pez406. Poult Sci. 2019. PMID: 31376349 Free PMC article.
-
Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-κB signaling pathway and NLRP3 inflammasome.Vet Res. 2020 Apr 10;51(1):52. doi: 10.1186/s13567-020-00777-x. Vet Res. 2020. PMID: 32276652 Free PMC article.
-
Molecular detection and genetic characterization of Mycoplasma gallisepticum and Mycoplasma synoviae in selected chicken breeds in South Africa.BMC Infect Dis. 2024 Jun 5;24(1):562. doi: 10.1186/s12879-024-09437-3. BMC Infect Dis. 2024. PMID: 38840040 Free PMC article.
-
Transcriptional Profiling of the Chicken Tracheal Response to Virulent Mycoplasma gallisepticum Strain Rlow.Infect Immun. 2017 Sep 20;85(10):e00343-17. doi: 10.1128/IAI.00343-17. Print 2017 Oct. Infect Immun. 2017. PMID: 28739827 Free PMC article.
-
Mycoplasma ovipneumoniae induces sheep airway epithelial cell apoptosis through an ERK signalling-mediated mitochondria pathway.BMC Microbiol. 2016 Sep 23;16(1):222. doi: 10.1186/s12866-016-0842-0. BMC Microbiol. 2016. PMID: 27663303 Free PMC article.
References
-
- Sato S, Nonomura I, Shimizu F, Shoya S, Horiuchi T (1970) Mixed infection with Mycoplasma gallisepticum and the B1 strain of Newcastle disease virus in chickens. Natl Inst Anim Health Q (Tokyo) 10: 58–65. - PubMed
-
- Gaunson JE, Philip CJ, Whithear KG, Browning GF (2000) Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology 146 (Pt5): 1223–1229. - PubMed
-
- Stipkovits L, Egyed L, Palfi V, Beres A, Pitlik E, et al. (2012) Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol 41: 51–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

