Human glial chimeric mice reveal astrocytic dependence of JC virus infection
- PMID: 25401469
- PMCID: PMC4348956
- DOI: 10.1172/JCI76629
Human glial chimeric mice reveal astrocytic dependence of JC virus infection
Abstract
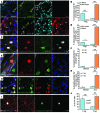
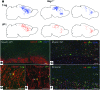
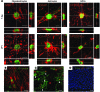
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease triggered by infection with the human gliotropic JC virus (JCV). Due to the human-selective nature of the virus, there are no animal models available to investigate JCV pathogenesis. To address this issue, we developed mice with humanized white matter by engrafting human glial progenitor cells (GPCs) into neonatal immunodeficient and myelin-deficient mice. Intracerebral delivery of JCV resulted in infection and subsequent demyelination of these chimeric mice. Human GPCs and astrocytes were infected more readily than oligodendrocytes, and viral replication was noted primarily in human astrocytes and GPCs rather than oligodendrocytes, which instead expressed early viral T antigens and exhibited apoptotic death. Engraftment of human GPCs in normally myelinated and immunodeficient mice resulted in humanized white matter that was chimeric for human astrocytes and GPCs. JCV effectively propagated in these mice, which indicates that astroglial infection is sufficient for JCV spread. Sequencing revealed progressive mutation of the JCV capsid protein VP1 after infection, suggesting that PML may evolve with active infection. These results indicate that the principal CNS targets for JCV infection are astrocytes and GPCs and that infection is associated with progressive mutation, while demyelination is a secondary occurrence, following T antigen-triggered oligodendroglial apoptosis. More broadly, this study provides a model by which to further assess the biology and treatment of human-specific gliotropic viruses.
Figures




Comment in
-
An animal model for progressive multifocal leukoencephalopathy.J Clin Invest. 2014 Dec;124(12):5103-6. doi: 10.1172/JCI79186. Epub 2014 Nov 17. J Clin Invest. 2014. PMID: 25401466 Free PMC article.
References
-
- Matoba T, et al. An siRNA against JC virus (JCV) agnoprotein inhibits JCV infection in JCV-producing cells inoculated in nude mice. Neuropathology. 2008;28(3):286–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

