Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations
- PMID: 25401470
- PMCID: PMC4348945
- DOI: 10.1172/JCI79100
Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations
Abstract
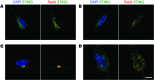
Innate immunity to viral infection involves induction of the type I IFN response; however, dysfunctional regulation of this pathway leads to inappropriate inflammation. Here, we evaluated a nonconsanguineous family of mixed European descent, with 4 members affected by systemic inflammatory and autoimmune conditions, including lupus, with variable clinical expression. We identified a germline dominant gain-of-function mutation in TMEM173, which encodes stimulator of type I IFN gene (STING), in the affected individuals. STING is a key signaling molecule in cytosolic DNA-sensing pathways, and STING activation normally requires dimerization, which is induced by 2'3' cyclic GMP-AMP (cGAMP) produced by the cGAMP synthase in response to cytosolic DNA. Structural modeling supported constitutive activation of the mutant STING protein based on stabilized dimerization. In agreement with the model predictions, we found that the STING mutant spontaneously localizes in the Golgi of patient fibroblasts and is constitutively active in the absence of exogenous 2'3'-cGAMP in vitro. Accordingly, we observed elevated serum IFN activity and a type I IFN signature in peripheral blood from affected family members. These findings highlight the key role of STING in activating both the innate and adaptive immune responses and implicate aberrant STING activation in features of human lupus.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

