Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation
- PMID: 25402209
- PMCID: PMC4277758
- DOI: 10.1021/ja508718m
Nickel-catalyzed Negishi arylations of propargylic bromides: a mechanistic investigation
Abstract
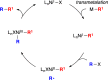
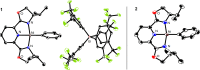
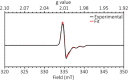
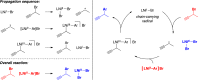
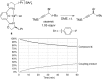
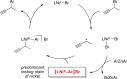
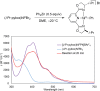
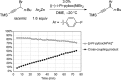
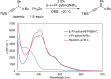
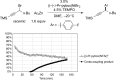
Although nickel-catalyzed stereoconvergent couplings of racemic alkyl electrophiles are emerging as a powerful tool in organic chemistry, to date there have been no systematic mechanistic studies of such processes. Herein, we examine the pathway for enantioselective Negishi arylations of secondary propargylic bromides, and we provide evidence for an unanticipated radical chain pathway wherein oxidative addition of the C-Br bond occurs through a bimetallic mechanism. In particular, we have crystallographically characterized a diamagnetic arylnickel(II) complex, [(i-Pr-pybox)Ni(II)Ph]BAr(F)4, and furnished support for [(i-Pr-pybox)Ni(II)Ph](+) being the predominant nickel-containing species formed under the catalyzed conditions as well as a key player in the cross-coupling mechanism. On the other hand, our observations do not require a role for an organonickel(I) intermediate (e.g., (i-Pr-pybox)Ni(I)Ph), which has previously been suggested to be an intermediate in nickel-catalyzed cross-couplings, oxidatively adding alkyl electrophiles through a monometallic pathway.
Figures

References
-
- For reviews and leading references, see:Science of Synthesis: Cross-Coupling and Heck-Type Reactions; Molander G. A., Ed.; Georg Thieme Verlag: Stuttgart, 2013.
-
- For leading references to mechanistic work on nickel-catalyzed cross-couplings of alkyl electrophiles, see:Hu X. Chem. Sci. 2011, 2, 1867–1886.
-
- Initial report:Zhou J.; Fu G. C. J. Am. Chem. Soc. 2003, 125, 14726–14727. - PubMed
- Initial report of stereoconvergent reactions:Fischer C.; Fu G. C. J. Am. Chem. Soc. 2005, 127, 4594–4595. - PubMed
- Most recent report of stereoconvergent reactions:Choi J.; Martín-Gago P.; Fu G. C. J. Am. Chem. Soc. 2014, 136, 12161–12165. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

