Enhanced gastrointestinal expression of cytosolic malic enzyme (ME1) induces intestinal and liver lipogenic gene expression and intestinal cell proliferation in mice
- PMID: 25402228
- PMCID: PMC4234650
- DOI: 10.1371/journal.pone.0113058
Enhanced gastrointestinal expression of cytosolic malic enzyme (ME1) induces intestinal and liver lipogenic gene expression and intestinal cell proliferation in mice
Abstract
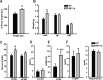
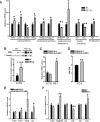
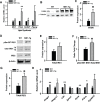
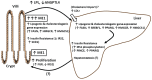
The small intestine participates in lipid digestion, metabolism and transport. Cytosolic malic enzyme 1 (ME1) is an enzyme that generates NADPH used in fatty acid and cholesterol biosynthesis. Previous work has correlated liver and adipose ME1 expression with susceptibility to obesity and diabetes; however, the contributions of intestine-expressed ME1 to these conditions are unknown. We generated transgenic (Tg) mice expressing rat ME1 in the gastrointestinal epithelium under the control of the murine villin1 promoter/enhancer. Levels of intestinal ME1 protein (endogenous plus transgene) were greater in Tg than wildtype (WT) littermates. Effects of elevated intestinal ME1 on body weight, circulating insulin, select adipocytokines, blood glucose, and metabolism-related genes were examined. Male Tg mice fed a high-fat (HF) diet gained significantly more body weight than WT male littermates and had heavier livers. ME1-Tg mice had deeper intestinal and colon crypts, a greater intestinal 5-bromodeoxyuridine labeling index, and increased expression of intestinal lipogenic (Fasn, Srebf1) and cholesterol biosynthetic (Hmgcsr, Hmgcs1), genes. The livers from HF diet-fed Tg mice also exhibited an induction of cholesterol and lipogenic pathway genes and altered measures (Irs1, Irs2, Prkce) of insulin sensitivity. Results indicate that gastrointestinal ME1 via its influence on intestinal epithelial proliferation, and lipogenic and cholesterologenic genes may concomitantly impact signaling in liver to modify this tissue's metabolic state. Our work highlights a new mouse model to address the role of intestine-expressed ME1 in whole body metabolism, hepatomegaly, and crypt cell proliferation. Intestinal ME1 may thus constitute a therapeutic target to reduce obesity-associated pathologies.
Conflict of interest statement
Figures



References
-
- de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, et al. (2008) The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics 1: 14 1755-8794-1-14 [pii];10.1186/1755-8794-1-14 [doi]. - DOI - PMC - PubMed
-
- Kondo H, Minegishi Y, Komine Y, Mori T, Matsumoto I, et al. (2006) Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am J Physiol Endocrinol Metab 291: E1092–E1099 00583.2005 [pii];10.1152/ajpendo.00583.2005 [doi]. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

