Azathioprine versus beta interferons for relapsing-remitting multiple sclerosis: a multicentre randomized non-inferiority trial
- PMID: 25402490
- PMCID: PMC4234663
- DOI: 10.1371/journal.pone.0113371
Azathioprine versus beta interferons for relapsing-remitting multiple sclerosis: a multicentre randomized non-inferiority trial
Abstract
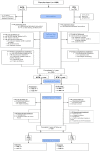
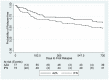
For almost three decades in many countries azathioprine has been used to treat relapsing-remitting multiple sclerosis. However its efficacy was usually considered marginal and following approval of β interferons for this indication it was no longer recommended as first line treatment, even if presently no conclusive direct β interferon-azathioprine comparison exists. To compare azathioprine efficacy versus the currently available β interferons in relapsing-remitting multiple sclerosis, a multicenter, randomized, controlled, single-blinded, non-inferiority trial was conducted in 30 Italian multiple sclerosis centers. Eligible patients (relapsing-remitting course; ≥ 2 relapses in the last 2 years) were randomly assigned to azathioprine or β interferons. The primary outcome was annualized relapse rate ratio (RR) over 2 years. Key secondary outcome was number of new brain MRI lesions. Patients (n = 150) were randomized in 2 groups (77 azathioprine, 73 β interferons). At 2 years, clinical evaluation was completed in 127 patients (62 azathioprine, 65 β interferons). Annualized relapse rate was 0.26 (95% Confidence Interval, CI, 0.19-0.37) in the azathioprine and 0.39 (95% CI 0.30-0.51) in the interferon group. Non-inferiority analysis showed that azathioprine was at least as effective as β interferons (relapse RRAZA/IFN 0.67, one-sided 95% CI 0.96; p<0.01). MRI outcomes were analyzed in 97 patients (50 azathioprine and 47 β interferons). Annualized new T2 lesion rate was 0.76 (95% CI 0.61-0.95) in the azathioprine and 0.69 (95% CI 0.54-0.88) in the interferon group. Treatment discontinuations due to adverse events were higher (20.3% vs. 7.8%, p = 0.03) in the azathioprine than in the interferon group, and concentrated within the first months of treatment, whereas in the interferon group discontinuations occurred mainly during the second year. The results of this study indicate that efficacy of azathioprine is not inferior to that of β interferons for patients with relapsing-remitting multiple sclerosis. Considering also the convenience of the oral administration, and the low cost for health service providers, azathioprine may represent an alternative to interferon treatment, while the different side effect profiles of both medications have to be taken into account.
Trial registration: EudraCT 2006-004937-13.
Conflict of interest statement
Figures
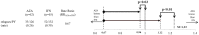

References
-
- The British, Dutch MSATG (1988) Double-masked trial of azathioprine in multiple sclerosis. british and dutch multiple sclerosis azathioprine trial group. Lancet 2: 179–183. - PubMed
-
- Ellison GW, Myers LW, Mickey MR, Graves MC, Tourtellotte WW, et al. (1989) A placebo-controlled, randomized, double-masked, variable dosage, clinical trial of azathioprine with and without methylprednisolone in multiple sclerosis. Neurology 39: 1018–1026. - PubMed
-
- Goodkin DE, Bailly RC, Teetzen ML, Hertsgaard D, Beatty WW (1991) The efficacy of azathioprine in relapsing-remitting multiple sclerosis. Neurology 41: 20–25. - PubMed
-
- Milanese C, La Mantia L, Salmaggi A, Eoli M (1993) A double blind study on azathioprine efficacy in multiple sclerosis: Final report. J Neurol 240: 295–298. - PubMed
-
- Clegg A, Bryant J, Milne R (2000) Disease-modifying drugs for multiple sclerosis: A rapid and systematic review. Health Technol Assess 4: i–101, i-iv, 1-101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

