Catalytic diamination of olefins via N-N bond activation
- PMID: 25402963
- PMCID: PMC4270412
- DOI: 10.1021/ar500344t
Catalytic diamination of olefins via N-N bond activation
Abstract
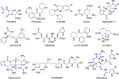
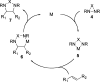
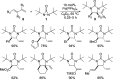
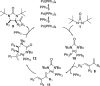
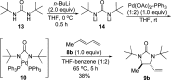
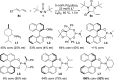
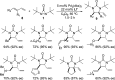
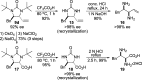
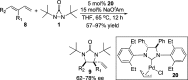
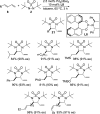
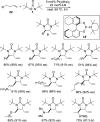
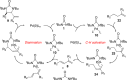
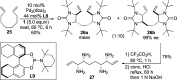
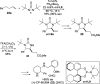
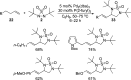
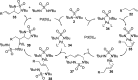
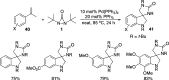
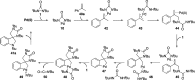
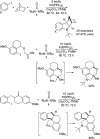
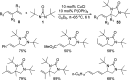
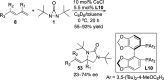
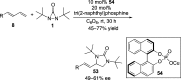
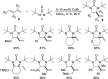
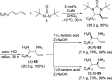
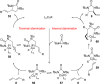
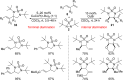
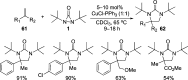
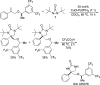
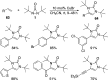
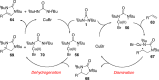
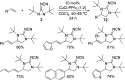
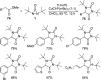
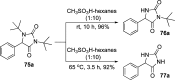
CONSPECTUS: Vicinal diamines are important structural motifs present in various biologically and chemically significant molecules. Direct diamination of olefins provides an effective approach to this class of compounds. Unlike well-established oxidation processes such as epoxidation, dihydroxylation, and aminohydroxylation, direct diamination of olefins had remained a long-standing challenge and had been less well developed. In this Account, we summarize our recent studies on Pd(0)- and Cu(I)-catalyzed diaminations of olefins using di-tert-butyldiaziridinone and its related analogues as nitrogen sources via N-N bond activation. A wide variety of imidazolidinones, cyclic sulfamides, indolines, imidazolinones, and cyclic guanidines can be obtained from conjugated dienes and terminal olefins. For conjugated dienes, the diamination proceeds regioselectively at the internal double bond with the Pd(0) catalyst. Mechanistic studies show that the diamination likely involves a four-membered Pd(II) species resulting from the insertion of Pd(0) into the N-N bond of di-tert-butyldiaziridinone. Interestingly, the Cu(I)-catalyzed process occurs regioselectively at either the terminal or internal double bond depending on the reaction conditions via two mechanistically distinct pathways. The Cu(I) catalyst cleaves the N-N bond of di-tert-butyldiaziridinone to form a Cu(II) nitrogen radical and a four-membered Cu(III) species, which are likely in rapid equilibrium. The Cu(II) nitrogen radical and the four-membered Cu(III) species lead to the terminal and internal diamination, respectively. Terminal olefins are effectively C-H diaminated at the allylic and homoallylic carbons with Pd(0) as catalyst and di-tert-butyldiaziridinone as nitrogen source, likely involving a diene intermediate generated in situ from the terminal olefin via formation of a π-allyl Pd complex and subsequent β-hydride elimination. When di-tert-butylthiadiaziridine 1,1-dioxide is used as nitrogen source, cyclic sulfamides are installed at the terminal carbons via a dehydrogenative diamination process. When α-methylstyrenes (lacking homoallylic hydrogens) react with Pd(0) and di-tert-butyldiaziridinone, spirocyclic indolines are formed with generation of four C-N bonds and one spiro quaternary carbon via allylic and aromatic C-H amination. With Cu(I) catalysts, various terminal olefins can be effectively diaminated at the double bonds using di-tert-butyldiaziridinone, di-tert-butylthiadiaziridine 1,1-dioxide, and 1,2-di-tert-butyl-3-(cyanimino)-diaziridine as nitrogen sources, giving a variety of imidazolidinones, cyclic sulfamides, and cyclic guanidines in good yields, respectively. In the case of monosubstituted olefins using di-tert-butyldiaziridinone as nitrogen source, the resulting diamination products (imidazolidinones) are readily dehydrogenated under the reaction conditions, leading to the corresponding imidazolinones in good yields. Esters can also be diaminated to form the corresponding hydantoins with di-tert-butyldiaziridinone in the presence of a Cu(I) catalyst. A radical mechanism is likely to be operating in these Cu(I)-catalyzed reaction processes. Asymmetric processes have also been developed for the Pd(0)- and Cu(I)-catalyzed diamination reactions. Biologically active compounds such as (+)-CP-99,994 and Sch 425078 have been synthesized via the diamination processes. The diamination reactions described herein provide efficient methods to access a wide variety of vicinal diamines from readily available olefins and show great potential for synthetic applications.
Figures
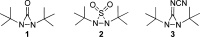
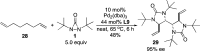
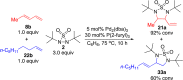
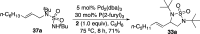
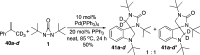
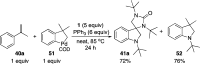
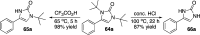
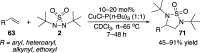
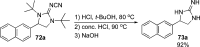
Similar articles
-
Cu(I)-catalyzed sequential diamination and dehydrogenation of terminal olefins: a facile approach to imidazolinones.Chemistry. 2014 Oct 20;20(43):13901-4. doi: 10.1002/chem.201404381. Epub 2014 Sep 11. Chemistry. 2014. PMID: 25213994 Free PMC article.
-
Synthetic and mechanistic studies on Pd(0)-catalyzed diamination of conjugated dienes.J Am Chem Soc. 2010 Mar 17;132(10):3523-32. doi: 10.1021/ja909459h. J Am Chem Soc. 2010. PMID: 20166669 Free PMC article.
-
Cu(I)-catalyzed diamination of conjugated dienes. Complementary regioselectivity from two distinct mechanistic pathways involving Cu(II) and Cu(III) species.J Am Chem Soc. 2011 Dec 28;133(51):20890-900. doi: 10.1021/ja207691a. Epub 2011 Dec 2. J Am Chem Soc. 2011. PMID: 22081888 Free PMC article.
-
Transition-Metal-Catalyzed 1,2-Diaminations of Olefins: Synthetic Methodologies and Mechanistic Studies.Chem Asian J. 2024 Jan 2;19(1):e202300705. doi: 10.1002/asia.202300705. Epub 2023 Dec 14. Chem Asian J. 2024. PMID: 37743249 Review.
-
Palladium-catalyzed allylic C-H bond functionalization of olefins.Top Curr Chem. 2010;292:195-209. doi: 10.1007/128_2009_16. Top Curr Chem. 2010. PMID: 21500407 Review.
Cited by
-
Stereoselective and Regioselective Synthesis of Heterocycles via Copper-Catalyzed Additions of Amine Derivatives and Alcohols to Alkenes.J Org Chem. 2017 Nov 3;82(21):11311-11325. doi: 10.1021/acs.joc.7b02072. Epub 2017 Sep 29. J Org Chem. 2017. PMID: 28910106 Free PMC article.
-
Synthesis of Unsymmetrical Vicinal Diamines via Directed Hydroamination.Org Lett. 2022 Aug 5;24(30):5513-5518. doi: 10.1021/acs.orglett.2c01911. Epub 2022 Jul 21. Org Lett. 2022. PMID: 35862860 Free PMC article.
-
Rh(III)-catalyzed Intra- and Intermolecular 3,4-Difunctionalization of 1,3-Dienes via Rh(III)-π-allyl Amidation with 1,4,2-Dioxazolones.ACS Catal. 2022 Aug 5;12(15):9690-9697. doi: 10.1021/acscatal.2c02537. Epub 2022 Jul 26. ACS Catal. 2022. PMID: 37829170 Free PMC article.
-
Access to Chiral Diamine Derivatives through Stereoselective Cu-Catalyzed Reductive Coupling of Imines and Allenamides.J Org Chem. 2021 Apr 2;86(7):5026-5046. doi: 10.1021/acs.joc.0c02971. Epub 2021 Mar 16. J Org Chem. 2021. PMID: 33724828 Free PMC article.
-
Brønsted acid-promoted hydroamination of unsaturated hydrazones: access to biologically important 5-arylpyrazolines.RSC Adv. 2021 May 11;11(28):17340-17345. doi: 10.1039/d1ra03043d. eCollection 2021 May 6. RSC Adv. 2021. PMID: 35479684 Free PMC article.
References
-
-
For leading reviews, see:
- Michalson E. T.; Szmuszkovicz J. Medicinal Agents Incorporating the 1,2-Diamine Functionality. Prog. Drug Res. 1989, 33, 135–149. - PubMed
- Lucet D.; Gall T. L.; Mioskowski C. The Chemistry of Vicinal Diamines. Angew. Chem., Int. Ed. 1998, 37, 2580–2627. - PubMed
- Kotti S. R. S. S.; Timmons C.; Li G. Vicinal Diamino Functionalities as Privileged Structural Elements in Biologically Active Compounds and Exploitation of Their Synthetic Chemistry. Chem. Biol. Drug Des. 2006, 67, 101–114. - PubMed
- Cardona F.; Goti A. Metal-Catalysed 1,2-Diamination Reactions. Nat. Chem. 2009, 1, 269–275. - PubMed
- Jong S. D.; Nosal D. G.; Wardrop D. J. Methods for Direct Alkene Diamination, New & Old. Tetrahedron 2012, 68, 4067–4105. - PMC - PubMed
-
-
-
For leading references, see:
- Shigematsu N.; Setoi H.; Uchida I.; Shibata T.; Terano H.; Hashimoto M. Structure and Synthesis of FR900490, a New Immunomodulating Peptide Isolated from a Fungus. Tetrahedron Lett. 1988, 29, 5147–5150.
- Desai M. C.; Lefkowitz S. L.; Thadeio P. F.; Longo K. P.; Snider R. M. Discovery of a Potent Substance P Antagonist: Recognition of the Key Molecular Determinant. J. Med. Chem. 1992, 35, 4911–4913. - PubMed
- Kinnel R. B.; Gehrken H.-P.; Scheuer P. J. Palau’amine: A Cytotoxic and Immunosuppressive Hexacyclic Bisguanidine Antibiotic from the Sponge Stylotella agminata. J. Am. Chem. Soc. 1993, 115, 3376–3377.
- Kuwahara A.; Nishikiori T.; Shimada N.; Nakagawa T.; Fukazawa H.; Mizuno S.; Uehara Y. NA22598A1, a Novel Antitumor Substance Produced by Streptomyces sp. NA22598. J. Antibiot. 1997, 50, 712–713. - PubMed
- Hanessian S.; Bayrakdarian M.; Luo X. Total Synthesis of A-315675: A Potent Inhibitor of Influenza Neuraminidase. J. Am. Chem. Soc. 2002, 124, 4716–4721. - PubMed
- Nishimura S.; Matsunaga S.; Shibazaki M.; Suzuki K.; Furihata K.; van Soest R. W. M.; Fusetani N. Massadine, a Novel Geranylgeranyltransferase Type I Inhibitor from the Marine Sponge Stylissa aff. massa. Org. Lett. 2003, 5, 2255–2257. - PubMed
-
-
- Kizirian J.-C. Chiral Tertiary Diamines in Asymmetric Synthesis. Chem. Rev. 2008, 108, 140–205. - PubMed
-
-
For leading reviews on diamination of olefins, see:
- Gasc M. B.; Lattes A.; Perie J. J. Amination of Alkenes. Tetrahedron 1983, 39, 703–731.
- Mortensen M. S.; O’Doherty G. A. Recent Advances in 1,2-Diamination of Alkenes. Chemtracts: Org. Chem. 2005, 18, 555–561.
- Muñiz K. The Development of Asymmetric Diamination of Alkenes with Imido-Osmium Reagents. New J. Chem. 2005, 29, 1371–1385.
- Jensen K. H.; Sigman M. S. Mechanistic Approaches to Palladium-Catalyzed Alkene Difunctionalization Reactions. Org. Biomol. Chem. 2008, 6, 4083–4088. - PMC - PubMed
- de Figueiredo R. M. Transition-Metal-Catalyzed Diamination of Olefins. Angew. Chem., Int. Ed. 2009, 48, 1190–1193. - PubMed
- Chemler S. R. Evolution of Copper(II) as a New Alkene Amination Promoter and Catalyst. J. Organomet. Chem. 2011, 696, 150–158. - PMC - PubMed
- Muñiz K.; Martínez C. Development of Intramolecular Vicinal Diamination of Alkenes: From Palladium to Bromine Catalysis. J. Org. Chem. 2013, 78, 2168–2174. - PubMed
-
-
-
For leading references on metal-mediated diamination, see: Tl,
- Aranda V. G.; Barluenga J.; Aznar F. The Addition of Aromatic Amines to Alkenes in the Presence of Thallium(III) Acetate. Synthesis 1974, 504–505.
-
Os,
- Chong A. O.; Oshima K.; Sharpless K. B. Synthesis of Dioxobis(tert-alkylimido)osmium(VIII) and Oxotris(tert-alkylimido)osmium(VIII) Complexes. Stereospecific Vicinal Diamination of Olefins. J. Am. Chem. Soc. 1977, 99, 3420–3426.
- Muñiz K.; Nieger M.; Mansikkamäki H. Stereochemically Defined Osmium Centers from Asymmetric Diamination of Olefins: Mechanistic Implications for Osmium-Mediated Acrylate Functionalization. Angew. Chem., Int. Ed. 2003, 42, 5958–5961. - PubMed
-
Pd,
- Bäckvall J.-E. Stereospecific Palladium-Promoted Vicinal Diamination of Olefins. Tetrahedron Lett. 1978, 19, 163–166.
-
Hg,
- Barluenga J.; Alonso-Cires L.; Asensio G. Mercury(II) Oxide/Tetrafluoroboric Acid – A New Reagent in Organic Synthesis; A Convenient Diamination of Olefins. Synthesis 1979, 962–964.
-
Co,
- Becker P. N.; White M. A.; Bergman R. G. A New Method for 1,2-Diamination of Alkenes Using Cyclopentadienylnitrosylcobalt Dimer/NO/LiAlH4. J. Am. Chem. Soc. 1980, 102, 5676–5677.
-
Mn,
- Fristad W. E.; Brandvold T. A.; Peterson J. R.; Thompson S. R. Conversion of Alkenes to 1,2-Diazides and 1,2-Diamines. J. Org. Chem. 1985, 50, 3647–3649.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

