Radioiodinated DPA-713 imaging correlates with bactericidal activity of tuberculosis treatments in mice
- PMID: 25403669
- PMCID: PMC4291409
- DOI: 10.1128/AAC.04180-14
Radioiodinated DPA-713 imaging correlates with bactericidal activity of tuberculosis treatments in mice
Abstract
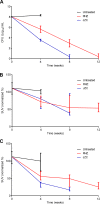
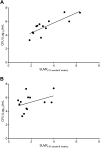
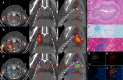
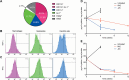
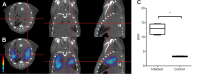
Current tools for monitoring response to tuberculosis treatments have several limitations. Noninvasive biomarkers could accelerate tuberculosis drug development and clinical studies, but to date little progress has been made in developing new imaging technologies for this application. In this study, we developed pulmonary single-photon emission computed tomography (SPECT) using radioiodinated DPA-713 to serially monitor the activity of tuberculosis treatments in live mice, which develop necrotic granulomas and cavitary lesions. C3HeB/FeJ mice were aerosol infected with Mycobacterium tuberculosis and administered either a standard or a highly active bedaquiline-containing drug regimen. Serial (125)I-DPA-713 SPECT imaging was compared with (18)F-fluorodeoxyglucose ((18)F-FDG) positron emission tomography (PET) and standard microbiology. Ex vivo studies were performed to characterize and correlate DPA-713 imaging with cellular and cytokine responses. Pulmonary (125)I-DPA-713 SPECT, but not (18)F-FDG PET, was able to correctly identify the bactericidal activities of the two tuberculosis treatments as early as 4 weeks after the start of treatment (P < 0.03). DPA-713 readily penetrated the fibrotic rims of necrotic and cavitary lesions. A time-dependent decrease in both tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) levels was observed with treatments, with (125)I-DPA-713 SPECT correlating best with tissue TNF-α levels (ρ = 0.94; P < 0.01). (124)I-DPA-713 was also evaluated as a PET probe and demonstrated a 4.0-fold-higher signal intensity in the infected tuberculous lesions than uninfected controls (P = 0.03). These studies provide proof of concept for application of a novel noninvasive imaging biomarker to monitor tuberculosis treatments, with the potential for application for humans.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi:10.1056/NEJMoa0907847. - DOI - PMC - PubMed
-
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. 2009. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136:420–425. doi:10.1378/chest.08-2427. - DOI - PubMed
-
- Davis SL, Nuermberger EL, Um PK, Vidal C, Jedynak B, Pomper MG, Bishai WR, Jain SK. 2009. Noninvasive pulmonary [18F]-2-fluoro-deoxy-d-glucose positron emission tomography correlates with bactericidal activity of tuberculosis drug treatment. Antimicrob Agents Chemother 53:4879–4884. doi:10.1128/AAC.00789-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

