Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2
- PMID: 25403771
- PMCID: PMC4599878
- DOI: 10.1111/pbi.12278
Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2
Abstract
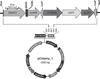
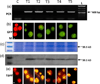
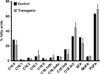
Higher lipid biosynthesis and accumulation are important to achieve economic viability of biofuel production via microalgae. To enhance lipid content, Chlamydomonas reinhardtii was genetically engineered with a key enzyme diacylglycerol acyltransferase (BnDGAT2) from Brassica napus, responsible for neutral lipid biosynthesis. The transformed colonies harbouring aph7 gene, screened on hygromycin-supplemented medium, achieved transformation frequency of ~120 ± 10 colonies/1 × 10(6) cells. Transgene integration and expression were confirmed by PCR, Southern blots, staining lipid droplets, proteins and spectro-fluorometric analysis of Nile red-stained cells. The neutral lipid is a major class (over 80% of total lipids) and most significant requirement for biodiesel production; this was remarkably higher in the transformed alga than the untransformed control. The levels of saturated fatty acids in the transformed alga decreased to about 7% while unsaturated fatty acids increased proportionately when compared to wild type cells. Polyunsaturated fatty acids, especially α-linolenic acid, an essential omega-3 fatty acid, were enhanced up to 12% in the transformed line. Nile red staining confirmed formation of a large number of lipid globules in the transformed alga. Evaluation of long-term stability and vitality of the transgenic alga revealed that cryopreservation produced significantly higher quantity of lipid than those maintained continuously over 128 generations on solid medium. The overexpression of BnDGAT2 significantly altered the fatty acids profile in the transformed alga. Results of this study offer a valuable strategy of genetic manipulation for enhancing polyunsaturated fatty acids and neutral lipids for biofuel production in algae.
Keywords: biodiesel; bioenergy; green algae; neutral lipids; polyunsaturated fatty acids; transgenic algae.
© 2014 Society for Experimental Biology, Association of Applied Biologists and John Wiley & Sons Ltd.
Figures

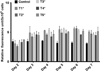

References
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959;37:911–917. - PubMed
-
- Boyle NR, Page MD, Liu B, Blaby IK, Casero D, Kropat J, Cokus SJ, Hong-Hermesdorf A, Shaw J, Karpowicz SJ. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J. Biol. Chem. 2012;287:15811–15825. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

