Extracellular excystation and development of Cryptosporidium: tracing the fate of oocysts within Pseudomonas aquatic biofilm systems
- PMID: 25403949
- PMCID: PMC4236811
- DOI: 10.1186/s12866-014-0281-8
Extracellular excystation and development of Cryptosporidium: tracing the fate of oocysts within Pseudomonas aquatic biofilm systems
Abstract
Background: Aquatic biofilms often serve as environmental reservoirs for microorganisms and provide them with a nutrient-rich growth environment under harsh conditions. With regard to Cryptosporidium, biofilms can serve as environmental reservoirs for oocysts, but may also support the growth of additional Cryptosporidium stages.
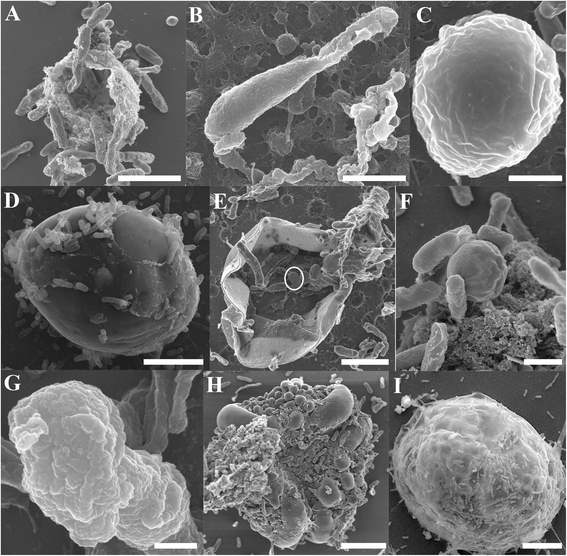
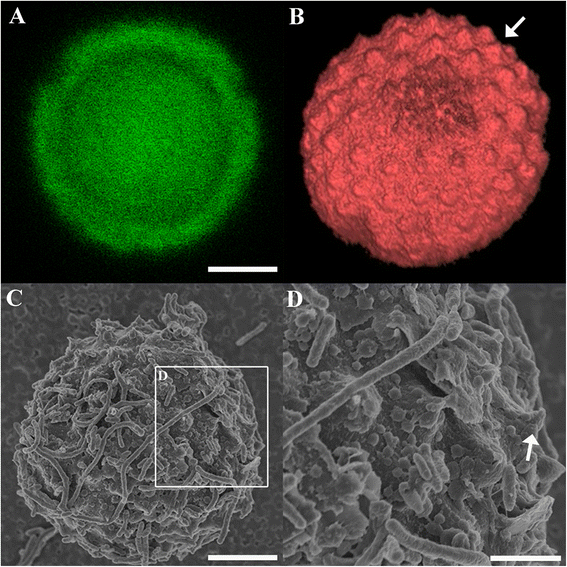
Results: Here we used confocal laser scanning microscopy, scanning electron microscopy (SEM), and flow cytometry to identify and describe various Cryptosporidium developmental stages present within aquatic biofilm systems, and to directly compare these to stages produced in cell culture. We also show that Cryptosporidium has the ability to form a parasitophorous vacuole independently, in a host-free biofilm environment, potentially allowing them to complete an extracellular life cycle. Correlative data from confocal and SEM imaging of the same cells confirmed that the observed developmental stages (including trophozoites, meronts, and merozoites) were Cryptosporidium. These microscopy observations were further supported by flow cytometric analyses, where excysted oocyst populations were detected in 1, 3 and 6 day-old Cryptosporidium-exposed biofilms, but not in biofilm-free controls.
Conclusions: These observations not only highlight the risk that aquatic biofilms pose in regards to Cryptosporidium outbreaks from water distribution systems, but further indicate that even simple biofilms are able to stimulate oocyst excystation and support the extracellular multiplication and development of Cryptosporidium within aquatic environments.
Figures






References
-
- Rogers J, Keevil C. Survival of Cryptosporidium parvum oocysts in biofilm and planktonic samples in a model system. In: Betts W, Casemore D, Fricker C, Smith H, Watkins J, editors. Protozoan parasites and water. Cambridge: The Royal Society of Chemistry; 1995.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

