The connexin46 mutant, Cx46T19M, causes loss of gap junction function and alters hemi-channel gating
- PMID: 25404239
- PMCID: PMC4300453
- DOI: 10.1007/s00232-014-9752-y
The connexin46 mutant, Cx46T19M, causes loss of gap junction function and alters hemi-channel gating
Abstract
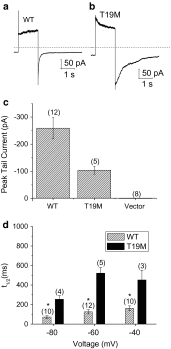
An N-terminal mutant of connexin46 (T19M) alters a highly conserved threonine and has been linked to autosomal dominant cataracts. To study the cellular and functional consequences of substitution of this amino acid, T19M was expressed in Xenopus oocytes and in HeLa cells. Unlike wild-type Cx46, T19M did not induce intercellular conductances in Xenopus oocytes. In transfected HeLa cells, T19M was largely localized within the cytoplasm, with drastically reduced formation of gap junction plaques. Expression of rat T19M was cytotoxic, as evidenced by an almost complete loss of viable cells expressing the mutant protein by 48-72 h following transfection. When incubated in medium containing physiological concentrations of divalent cations, T19M-expressing cells showed increased uptake of DAPI as compared with cells expressing wild-type Cx46, suggesting aberrant connexin hemi-channel activity. Time-lapse and dye uptake studies suggested that T19M hemi-channels had reduced sensitivity to Ca(2+). Whole cell patch clamp studies of single transfected HeLa cells demonstrated that rat T19M formed functional hemi-channels with altered voltage-dependent gating. These data suggest that T19M causes cataracts by loss of gap junctional channel function and abnormally increased hemi-channel activity. Furthermore, they implicate this conserved threonine in both gap junction plaque formation and channel/hemi-channel gating in Cx46.
Figures







References
-
- Arora A, Minogue PJ, Liu X, Reddy MA, Ainsworth JR, Bhattacharya SS, Webster AR, Hunt DM, Ebihara L, Moore AT, Beyer EC, Berthoud VM. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43:e2. doi: 10.1136/jmg.2005.034108. - DOI - PMC - PubMed
-
- Beyer EC, Berthoud VM. The family of connexin genes. In: Harris AL, Locke D, editors. Connexins: a guide. New York: Humana Press; 2009. pp. 3–26.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

