Melanopsin mediates light-dependent relaxation in blood vessels
- PMID: 25404319
- PMCID: PMC4273372
- DOI: 10.1073/pnas.1420258111
Melanopsin mediates light-dependent relaxation in blood vessels
Abstract
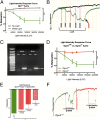
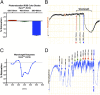
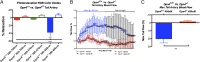
Melanopsin (opsin4; Opn4), a non-image-forming opsin, has been linked to a number of behavioral responses to light, including circadian photo-entrainment, light suppression of activity in nocturnal animals, and alertness in diurnal animals. We report a physiological role for Opn4 in regulating blood vessel function, particularly in the context of photorelaxation. Using PCR, we demonstrate that Opn4 (a classic G protein-coupled receptor) is expressed in blood vessels. Force-tension myography demonstrates that vessels from Opn4(-/-) mice fail to display photorelaxation, which is also inhibited by an Opn4-specific small-molecule inhibitor. The vasorelaxation is wavelength-specific, with a maximal response at ∼430-460 nm. Photorelaxation does not involve endothelial-, nitric oxide-, carbon monoxide-, or cytochrome p450-derived vasoactive prostanoid signaling but is associated with vascular hyperpolarization, as shown by intracellular membrane potential measurements. Signaling is both soluble guanylyl cyclase- and phosphodiesterase 6-dependent but protein kinase G-independent. β-Adrenergic receptor kinase 1 (βARK 1 or GRK2) mediates desensitization of photorelaxation, which is greatly reduced by GRK2 inhibitors. Blue light (455 nM) regulates tail artery vasoreactivity ex vivo and tail blood blood flow in vivo, supporting a potential physiological role for this signaling system. This endogenous opsin-mediated, light-activated molecular switch for vasorelaxation might be harnessed for therapy in diseases in which altered vasoreactivity is a significant pathophysiologic contributor.
Keywords: GRK2; melanopsin; opsin; photorelaxation; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
PDE and sGC hand in hand to see the light.Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17704-5. doi: 10.1073/pnas.1421161111. Epub 2014 Dec 2. Proc Natl Acad Sci U S A. 2014. PMID: 25468979 Free PMC article. No abstract available.
References
-
- Furchgott RF, Sleator WJ, Mccaman MW, Elchlepp J. Relaxation of arterial strips by light and the influence of drugs on this photodynamic effect. J Pharmacol Exp Ther. 1955;113:22.
-
- Matsunaga K, Furchgott RF. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther. 1989;248(2):687–695. - PubMed
-
- Chen X, Gillis CN. Enhanced photorelaxation in aorta, pulmonary artery and corpus cavernosum produced by BAY K 8644 or N-nitro-L-arginine. Biochem Biophys Res Commun. 1992;186(3):1522–1527. - PubMed
-
- Andrews KL, Triggle CR, Ellis A. NO and the vasculature: Where does it come from and what does it do? Heart Fail Rev. 2002;7(4):423–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

